Randomized, double-blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy
- PMID: 23847109
- PMCID: PMC4237123
- DOI: 10.1002/hep.26611
Randomized, double-blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy
Abstract
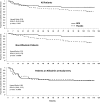
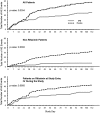
Glycerol phenylbutyrate (GPB) lowers ammonia by providing an alternate pathway to urea for waste nitrogen excretion in the form of phenylacetyl glutamine, which is excreted in urine. This randomized, double-blind, placebo-controlled phase II trial enrolled 178 patients with cirrhosis, including 59 already taking rifaximin, who had experienced two or more hepatic encephalopathy (HE) events in the previous 6 months. The primary endpoint was the proportion of patients with HE events. Other endpoints included the time to first event, total number of events, HE hospitalizations, symptomatic days, and safety. GPB, at 6 mL orally twice-daily, significantly reduced the proportion of patients who experienced an HE event (21% versus 36%; P=0.02), time to first event (hazard ratio [HR]=0.56; P<0.05), as well as total events (35 versus 57; P=0.04), and was associated with fewer HE hospitalizations (13 versus 25; P=0.06). Among patients not on rifaximin at enrollment, GPB reduced the proportion of patients with an HE event (10% versus 32%; P<0.01), time to first event (HR=0.29; P<0.01), and total events (7 versus 31; P<0.01). Plasma ammonia was significantly lower in patients on GPB and correlated with HE events when measured either at baseline or during the study. A similar proportion of patients in the GPB (79%) and placebo groups (76%) experienced adverse events.
Conclusion: GPB reduced HE events as well as ammonia in patients with cirrhosis and HE and its safety profile was similar to placebo. The findings implicate ammonia in the pathogenesis of HE and suggest that GPB has therapeutic potential in this population. (Clinicaltrials.gov, NCT00999167).
© 2014 The Authors. Hepatology published by Wiley on behalf of the American Association for the Study of Liver Diseases.
Figures


Comment in
-
Drug-induced removal of nitrogen derivatives in urine: a new concept whose time has come.Hepatology. 2014 Mar;59(3):764-6. doi: 10.1002/hep.26789. Epub 2014 Jan 27. Hepatology. 2014. PMID: 24123173 No abstract available.
-
New strategies for treating hepatic encephalopathy.Ann Hepatol. 2014 May-Jun;13(3):409-11. Ann Hepatol. 2014. PMID: 24756021 No abstract available.
References
-
- Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl. J Med. 2010;362:1071–1081. - PubMed
-
- Cordoba J, Minguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70–80. - PubMed
-
- Mullen KD, Ferenci P, Bass NM, Leevy CV, Keeffe EB. An algorithm for the management of hepatic encephalopathy. Semin Liver Dis. 2007;27(Suppl 2):32–48.
-
- Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188–193. - PubMed
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
