Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis
- PMID: 23847135
- PMCID: PMC3796013
- DOI: 10.1002/stem.1460
Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis
Abstract
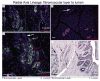
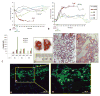
Peribiliary glands (PBGs) in bile duct walls, and pancreatic duct glands (PDGs) associated with pancreatic ducts, in humans of all ages, contain a continuous, ramifying network of cells in overlapping maturational lineages. We show that proximal (PBGs)-to-distal (PDGs) maturational lineages start near the duodenum with cells expressing markers of pluripotency (NANOG, OCT4, and SOX2), proliferation (Ki67), self-replication (SALL4), and early hepato-pancreatic commitment (SOX9, SOX17, PDX1, and LGR5), transitioning to PDG cells with no expression of pluripotency or self-replication markers, maintenance of pancreatic genes (PDX1), and expression of markers of pancreatic endocrine maturation (NGN3, MUC6, and insulin). Radial-axis lineages start in PBGs near the ducts' fibromuscular layers with stem cells and end at the ducts' lumens with cells devoid of stem cell traits and positive for pancreatic endocrine genes. Biliary tree-derived cells behaved as stem cells in culture under expansion conditions, culture plastic and serum-free Kubota's Medium, proliferating for months as undifferentiated cells, whereas pancreas-derived cells underwent only approximately 8-10 divisions, then partially differentiated towards an islet fate. Biliary tree-derived cells proved precursors of pancreas' committed progenitors. Both could be driven by three-dimensional conditions, islet-derived matrix components and a serum-free, hormonally defined medium for an islet fate (HDM-P), to form spheroids with ultrastructural, electrophysiological and functional characteristics of neoislets, including glucose regulatability. Implantation of these neoislets into epididymal fat pads of immunocompromised mice, chemically rendered diabetic, resulted in secretion of human C-peptide, regulatable by glucose, and able to alleviate hyperglycemia in hosts. The biliary tree-derived stem cells and their connections to pancreatic committed progenitors constitute a biological framework for life-long pancreatic organogenesis.
Keywords: biliary tree stem cells; maturational lineages; organogenesis; pancreas; pancreatic duct glands; peribiliary glands.
© AlphaMed Press.
Figures








References
-
- Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–148. - PubMed
-
- Dor Y, Brown J, Martinez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
-
- Zhao M, Amiel SA, Christie MR, et al. Evidence for the presence of stem cell-like progenitor cells in human adult pancreas. Journal of Endocrinology. 2007;195:407–414. - PubMed
-
- Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

