Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma
- PMID: 23847203
- PMCID: PMC3732970
- DOI: 10.1073/pnas.1307218110
Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma
Abstract
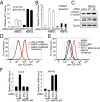
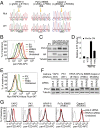
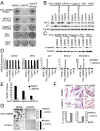
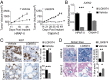
A growing number of agents targeting ligand-induced Wnt/β-catenin signaling are being developed for cancer therapy. However, clinical development of these molecules is challenging because of the lack of a genetic strategy to identify human tumors dependent on ligand-induced Wnt/β-catenin signaling. Ubiquitin E3 ligase ring finger 43 (RNF43) has been suggested as a negative regulator of Wnt signaling, and mutations of RNF43 have been identified in various tumors, including cystic pancreatic tumors. However, loss of function study of RNF43 in cell culture has not been conducted, and the functional significance of RNF43 mutations in cancer is unknown. Here, we show that RNF43 inhibits Wnt/β-catenin signaling by reducing the membrane level of Frizzled in pancreatic cancer cells, serving as a negative feedback mechanism. Inhibition of endogenous Wnt/β-catenin signaling increased the cell surface level of Frizzled. A panel of 39 pancreatic cancer cell lines was tested for Wnt dependency using LGK974, a selective Porcupine inhibitor being examined in a phase 1 clinical trial. Strikingly, all LGK974-sensitive lines carried inactivating mutations of RNF43. Inhibition of Wnt secretion, depletion of β-catenin, or expression of wild-type RNF43 blocked proliferation of RNF43 mutant but not RNF43-wild-type pancreatic cancer cells. LGK974 inhibited proliferation and induced differentiation of RNF43-mutant pancreatic adenocarcinoma xenograft models. Our data suggest that mutational inactivation of RNF43 in pancreatic adenocarcinoma confers Wnt dependency, and the presence of RNF43 mutations could be used as a predictive biomarker for patient selection supporting the clinical development of Wnt inhibitors in subtypes of cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol 20:781–810. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. - PubMed
-
- Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends Mol Med. 2012;18(8):483–493. - PubMed
-
- Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

