Theoretical analysis of vascular regulatory mechanisms contributing to retinal blood flow autoregulation
- PMID: 23847315
- PMCID: PMC3747715
- DOI: 10.1167/iovs.12-11543
Theoretical analysis of vascular regulatory mechanisms contributing to retinal blood flow autoregulation
Abstract
Purpose: To study whether impaired retinal autoregulation is a risk factor for glaucoma, the relationship between vascular regulatory mechanisms and glaucoma progression needs to be investigated. In this study, a vascular wall mechanics model is used to predict the relative importance of regulatory mechanisms in achieving retinal autoregulation.
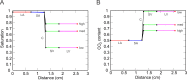
Methods: Resistance vessels are assumed to respond to changes in pressure, shear stress, carbon dioxide (CO2), and the downstream metabolic state communicated via conducted responses. Model parameters governing wall tension are fit to pressure and diameter data from porcine retinal arterioles. The autoregulation pressure range for control and elevated levels of IOP is predicted.
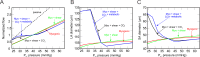
Results: The factor by which flow changes as the blood pressure exiting the central retinal artery is varied between 28 and 40 mm Hg is used to indicate the degree of autoregulation (1 indicates perfect autoregulation). In the presence of only the myogenic response mechanism, the factor is 2.06. In the presence of the myogenic and CO2 responses, the factor is 1.22. The combination of myogenic, shear, CO2, and metabolic responses yields the best autoregulation (factor of 1.10).
Conclusions: Model results are compared with flow and pressure data from multiple patient studies, and the combined effects of the metabolic and CO2 responses are predicted to be critical for achieving retinal autoregulation. When IOP is elevated, the model predicts a decrease in the autoregulation range toward low perfusion pressure, which is consistent with observations that glaucoma is associated with decreased perfusion pressure.
Keywords: autoregulation; blood flow regulation; glaucoma; metabolic response; myogenic response; retina.
Figures




References
-
- Leske MC. Open-angle glaucoma—an epidemiologic overview. Ophthalmic Epidemiol. 2007; 14: 166–172 - PubMed
-
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007; 114: 1965–1972 - PubMed
-
- Bengtsson B, Leske MC, Yang Z, Heijl A. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008; 115: 2044–2048 - PubMed
-
- De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011; 129: 562–568 - PubMed
-
- Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch Ophthalmol. 2007; 125: 805–812 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

