An automated method for identifying artifact in independent component analysis of resting-state FMRI
- PMID: 23847511
- PMCID: PMC3706880
- DOI: 10.3389/fnhum.2013.00343
An automated method for identifying artifact in independent component analysis of resting-state FMRI
Abstract
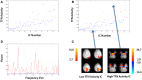
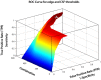
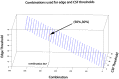
An enduring issue with data-driven analysis and filtering methods is the interpretation of results. To assist, we present an automatic method for identification of artifact in independent components (ICs) derived from functional MRI (fMRI). The method was designed with the following features: does not require temporal information about an fMRI paradigm; does not require the user to train the algorithm; requires only the fMRI images (additional acquisition of anatomical imaging not required); is able to identify a high proportion of artifact-related ICs without removing components that are likely to be of neuronal origin; can be applied to resting-state fMRI; is automated, requiring minimal or no human intervention. We applied the method to a MELODIC probabilistic ICA of resting-state functional connectivity data acquired in 50 healthy control subjects, and compared the results to a blinded expert manual classification. The method identified between 26 and 72% of the components as artifact (mean 55%). About 0.3% of components identified as artifact were discordant with the manual classification; retrospective examination of these ICs suggested the automated method had correctly identified these as artifact. We have developed an effective automated method which removes a substantial number of unwanted noisy components in ICA analyses of resting-state fMRI data. Source code of our implementation of the method is available.
Keywords: ICA; artifacts; automated classification; automatic; fMRI; functional magnetic resonance imaging; independent component analysis; independent component labeling.
Figures







Similar articles
-
Dimensionality of ICA in resting-state fMRI investigated by feature optimized classification of independent components with SVM.Front Hum Neurosci. 2015 May 8;9:259. doi: 10.3389/fnhum.2015.00259. eCollection 2015. Front Hum Neurosci. 2015. PMID: 26005413 Free PMC article.
-
Time course based artifact identification for independent components of resting-state FMRI.Front Hum Neurosci. 2013 May 23;7:214. doi: 10.3389/fnhum.2013.00214. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23734119 Free PMC article.
-
Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI.Neuroimage. 2015 May 15;112:278-287. doi: 10.1016/j.neuroimage.2015.02.063. Epub 2015 Mar 11. Neuroimage. 2015. PMID: 25770990
-
ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data.Neuroimage. 2015 May 15;112:267-277. doi: 10.1016/j.neuroimage.2015.02.064. Epub 2015 Mar 11. Neuroimage. 2015. PMID: 25770991
-
Evaluating the effects of systemic low frequency oscillations measured in the periphery on the independent component analysis results of resting state networks.Neuroimage. 2013 Aug 1;76:202-15. doi: 10.1016/j.neuroimage.2013.03.019. Epub 2013 Mar 21. Neuroimage. 2013. PMID: 23523805 Free PMC article.
Cited by
-
Personalized microstructural evaluation using a Mahalanobis-distance based outlier detection strategy on epilepsy patients' DTI data - Theory, simulations and example cases.PLoS One. 2019 Sep 23;14(9):e0222720. doi: 10.1371/journal.pone.0222720. eCollection 2019. PLoS One. 2019. PMID: 31545838 Free PMC article.
-
A robust classifier to distinguish noise from fMRI independent components.PLoS One. 2014 Apr 18;9(4):e95493. doi: 10.1371/journal.pone.0095493. eCollection 2014. PLoS One. 2014. PMID: 24748378 Free PMC article.
-
Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study.Hum Brain Mapp. 2015 Apr;36(4):1484-93. doi: 10.1002/hbm.22717. Epub 2014 Dec 11. Hum Brain Mapp. 2015. PMID: 25504964 Free PMC article.
-
Denoising the speaking brain: toward a robust technique for correcting artifact-contaminated fMRI data under severe motion.Neuroimage. 2014 Dec;103:33-47. doi: 10.1016/j.neuroimage.2014.09.013. Epub 2014 Sep 16. Neuroimage. 2014. PMID: 25225001 Free PMC article.
-
Intranasal oxytocin modulates the salience network in aging.Neuroimage. 2022 Jun;253:119045. doi: 10.1016/j.neuroimage.2022.119045. Epub 2022 Mar 5. Neuroimage. 2022. PMID: 35259525 Free PMC article. Clinical Trial.
References
-
- Abbott D., Jackson G. (2001). iBrain – software for analysis and visualisation of functional MR images. Neuroimage 13, S59.10.1016/S1053-8119(01)91402-8 - DOI
-
- Abbott D., Masterton R., Waites A., Bhaganagarapu K., Pell G., Harvey M., et al. (2011). “The iBrainTM analysis toolbox for SPM,” in Proceedings of the 17th Annual Meeting of the Organisation for Human Brain Mapping, Quebec City
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

