Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder
- PMID: 23847551
- PMCID: PMC3697031
- DOI: 10.3389/fpsyt.2013.00062
Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder
Abstract
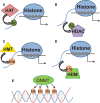
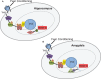
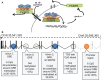
This review examines recent work on epigenetic mechanisms underlying animal models of fear learning as well as its translational implications in disorders of fear regulation, such as Post-traumatic Stress Disorder (PTSD). Specifically, we will examine work outlining roles of differential histone acetylation and DNA-methylation associated with consolidation, reconsolidation, and extinction in Pavlovian fear paradigms. We then focus on the numerous studies examining the epigenetic modifications of the Brain-derived neurotrophin factor (BDNF) pathway and the extension of these findings from animal models to recent work in human clinical populations. We will also review recently published data on FKBP5 regulation of glucocorticoid receptor function, and how this is modulated in animal models of PTSD and in human clinical populations via epigenetic mechanisms. As glucocorticoid regulation of memory consolidation is well established in fear models, we examine how these recent data contribute to our broader understanding of fear memory formation. The combined recent progress in epigenetic modulation of memory with the advances in fear neurobiology suggest that this area may be critical to progress in our understanding of fear-related disorders with implications for new approaches to treatment and prevention.
Keywords: PTSD; amygdala; biomarkers; consolidation; extinction; fear memory; reconsolidation.
Figures

References
-
- Alarcon J. M., Malleret G., Touzani K., Vronskaya S., Ishii S., Kandel E. R., et al. (2004). Chromatin acetylation, memory, and LTP are impaired in CBP±mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42, 947–95910.1016/j.neuron.2004.05.021 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

