The importance of tau phosphorylation for neurodegenerative diseases
- PMID: 23847585
- PMCID: PMC3696910
- DOI: 10.3389/fneur.2013.00083
The importance of tau phosphorylation for neurodegenerative diseases
Abstract
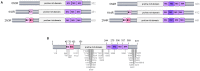
Fibrillar deposits of highly phosphorylated tau are a key pathological feature of several neurodegenerative tauopathies including Alzheimer's disease (AD) and some frontotemporal dementias. Increasing evidence suggests that the presence of these end-stage neurofibrillary lesions do not cause neuronal loss, but rather that alterations to soluble tau proteins induce neurodegeneration. In particular, aberrant tau phosphorylation is acknowledged to be a key disease process, influencing tau structure, distribution, and function in neurons. Although typically described as a cytosolic protein that associates with microtubules and regulates axonal transport, several additional functions of tau have recently been demonstrated, including roles in DNA stabilization, and synaptic function. Most recently, studies examining the trans-synaptic spread of tau pathology in disease models have suggested a potential role for extracellular tau in cell signaling pathways intrinsic to neurodegeneration. Here we review the evidence showing that tau phosphorylation plays a key role in neurodegenerative tauopathies. We also comment on the tractability of altering phosphorylation-dependent tau functions for therapeutic intervention in AD and related disorders.
Keywords: Alzheimer’s disease; extracellular; function; oligomers; phosphorylation; tau.
Figures

References
-
- Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol (2002) 61:547–56 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

