The Novel Actions of the Metabolite GnRH-(1-5) are Mediated by a G Protein-Coupled Receptor
- PMID: 23847594
- PMCID: PMC3703583
- DOI: 10.3389/fendo.2013.00083
The Novel Actions of the Metabolite GnRH-(1-5) are Mediated by a G Protein-Coupled Receptor
Abstract
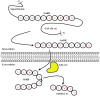
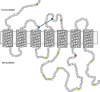
The gonadotropin-releasing hormone (GnRH) was originally isolated from the mammalian hypothalamus for its role as the primary regulator of reproductive function. Since its discovery, GnRH has also been shown to be located in non-hypothalamic tissues and is known to have diverse functions. Although the regulation of GnRH synthesis and release has been extensively studied, there is additional evidence to suggest that the processing of GnRH to the metabolite GnRH-(1-5) represents another layer of regulation. The focus of this review will be on the current evidence for the action of the pentapeptide metabolite GnRH-(1-5) in regulating cellular migration. We discuss the potential role of GnRH-(1-5) in regulating GnRH neuronal migration during development. Furthermore, we demonstrate these actions are mediated by the activation of a G protein-coupled receptor. Our findings suggest that GnRH-(1-5) may play a developmental function in addition to regulating developing cells.
Keywords: EP24.15; GPCR; GPR173; GnRH; SREB3; migration.
Figures


Similar articles
-
Regulation of Gonadotropin-Releasing Hormone-(1-5) Signaling Genes by Estradiol Is Age Dependent.Front Endocrinol (Lausanne). 2017 Oct 27;8:282. doi: 10.3389/fendo.2017.00282. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29163355 Free PMC article.
-
The metabolite GnRH-(1-5) inhibits the migration of immortalized GnRH neurons.Endocrinology. 2013 Feb;154(2):783-95. doi: 10.1210/en.2012-1746. Epub 2013 Jan 15. Endocrinology. 2013. PMID: 23321696
-
The role of GnRH metabolite, GnRH-(1-5), in endometrial cancer.Front Endocrinol (Lausanne). 2023 Apr 14;14:1183278. doi: 10.3389/fendo.2023.1183278. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37124730 Free PMC article. Review.
-
GnRH-(1-5) Inhibits TGF-β Signaling to Regulate the Migration of Immortalized Gonadotropin-Releasing Hormone Neurons.Front Endocrinol (Lausanne). 2018 Feb 20;9:45. doi: 10.3389/fendo.2018.00045. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29515521 Free PMC article.
-
A biological role for the gonadotrophin-releasing hormone (GnRH) metabolite, GnRH-(1-5).J Neuroendocrinol. 2009 Mar;21(4):293-8. doi: 10.1111/j.1365-2826.2009.01854.x. J Neuroendocrinol. 2009. PMID: 19210292 Review.
Cited by
-
Regulation of Gonadotropin-Releasing Hormone-(1-5) Signaling Genes by Estradiol Is Age Dependent.Front Endocrinol (Lausanne). 2017 Oct 27;8:282. doi: 10.3389/fendo.2017.00282. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29163355 Free PMC article.
-
Effect of hypothyroidism on the hypothalamic-pituitary-ovarian axis and reproductive function of pregnant rats.BMC Endocr Disord. 2018 May 24;18(1):30. doi: 10.1186/s12902-018-0258-y. BMC Endocr Disord. 2018. PMID: 29793475 Free PMC article.
-
Spatial and quantitative gene expression analysis of SREB receptors in the gonads of green-spotted pufferfish (Dichotomyctere nigroviridis).Gen Comp Endocrinol. 2025 Jan 1;360:114641. doi: 10.1016/j.ygcen.2024.114641. Epub 2024 Nov 12. Gen Comp Endocrinol. 2025. PMID: 39536984
-
New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides.Cells. 2022 Mar 10;11(6):957. doi: 10.3390/cells11060957. Cells. 2022. PMID: 35326408 Free PMC article. Review.
-
Phoenixin-A Pleiotropic Gut-Brain Peptide.Int J Mol Sci. 2018 Jun 11;19(6):1726. doi: 10.3390/ijms19061726. Int J Mol Sci. 2018. PMID: 29891773 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

