Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease
- PMID: 23847622
- PMCID: PMC3705591
- DOI: 10.3389/fimmu.2013.00181
Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease
Abstract
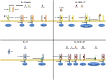
In addition to their well-established role(s) in the pathogenesis of gastrointestinal (GI)-related inflammatory disorders, including inflammatory bowel disease (IBD) and inflammation-associated colorectal cancer (CRC), emerging evidence confirms the critical involvement of the interleukin-1 (IL-1) cytokine family and their ligands in the maintenance of normal gut homeostasis. In fact, the paradigm that IBD occurs in two distinct phases is substantiated by the observation that classic IL-1 family members, such as IL-1, the IL-1 receptor antagonist (IL-1Ra), and IL-18, possess dichotomous functions depending on the phase of disease, as well as on their role in initiating vs. sustaining chronic gut inflammation. Another recently characterized IL-1 family member, IL-33, also possesses dual functions in the gut. IL-33 is upregulated in IBD and potently induces Th2 immune responses, while also amplifying Th1-mediated inflammation. Neutralization studies in acute colitis models, however, have yielded controversial results and recent reports suggest a protective role of IL-33 in epithelial regeneration and mucosal wound healing. Finally, although little is currently known regarding the potential contribution of IL-36 family members in GI inflammation/homeostasis, another IL-1 family member, IL-37, is emerging as a potent anti-inflammatory cytokine with the ability to down-regulate colitis. This new body of information has important translational implications for both the prevention and treatment of patients suffering from IBD and inflammation-associated CRC.
Keywords: IL-1 family of cytokines; Toll/IL-1 receptor family; colitis; inflammation-associated colon cancer; inflammatory bowel disease; intestinal fibrosis; intestinal homeostasis; mucosal wound healing.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

