Intersection of transfer cells with phloem biology-broad evolutionary trends, function, and induction
- PMID: 23847631
- PMCID: PMC3696738
- DOI: 10.3389/fpls.2013.00221
Intersection of transfer cells with phloem biology-broad evolutionary trends, function, and induction
Abstract
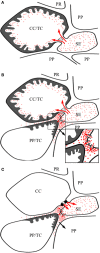
Transfer cells (TCs) are ubiquitous throughout the plant kingdom. Their unique ingrowth wall labyrinths, supporting a plasma membrane enriched in transporter proteins, provides these cells with an enhanced membrane transport capacity for resources. In certain plant species, TCs have been shown to function to facilitate phloem loading and/or unloading at cellular sites of intense resource exchange between symplasmic/apoplasmic compartments. Within the phloem, the key cellular locations of TCs are leaf minor veins of collection phloem and stem nodes of transport phloem. In these locations, companion and phloem parenchyma cells trans-differentiate to a TC morphology consistent with facilitating loading and re-distribution of resources, respectively. At a species level, occurrence of TCs is significantly higher in transport than in collection phloem. TCs are absent from release phloem, but occur within post-sieve element unloading pathways and particularly at interfaces between generations of developing Angiosperm seeds. Experimental accessibility of seed TCs has provided opportunities to investigate their inductive signaling, regulation of ingrowth wall formation and membrane transport function. This review uses this information base to explore current knowledge of phloem transport function and inductive signaling for phloem-associated TCs. The functional role of collection phloem and seed TCs is supported by definitive evidence, but no such information is available for stem node TCs that present an almost intractable experimental challenge. There is an emerging understanding of inductive signals and signaling pathways responsible for initiating trans-differentiation to a TC morphology in developing seeds. However, scant information is available to comment on a potential role for inductive signals (auxin, ethylene and reactive oxygen species) that induce seed TCs, in regulating induction of phloem-associated TCs. Biotic phloem invaders have been used as a model to speculate on involvement of these signals.
Keywords: inductive signals; ingrowth wall architecture; phloem transport; transfer cell.
Figures


References
-
- Aloni R. (2010). The induction of vascular tissues by auxin in ‘Plant Hormones. Biosynthesis, Signal Transduction, Action! ed Davies P. J. (Amsterdam: Springer; ), 485–518
-
- Aloni R., Schwalm K., Langhans M., Ullrich C. L. (2003). Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216, 841–853 - PubMed
-
- Andriunas F. A., Zhang H. M., Weber H., McCurdy D. W., Offler C. E., Patrick J. W. (2011). Glucose and ethylene signalling pathways converge to regulate trans-differentiation of epidermal transfer cells in Vicia narbonensis cotyledons. Plant J. 68, 987–998 10.1111/j.1365-313X.2011.04749.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

