Therapeutic targeting of NOD1 receptors
- PMID: 23848281
- PMCID: PMC3791987
- DOI: 10.1111/bph.12300
Therapeutic targeting of NOD1 receptors
Abstract
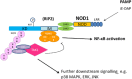
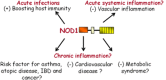
The nucleotide-binding oligomerization domain 1 (NOD1) protein is an intracellular receptor for breakdown products of peptidoglycan (PGN), an essential bacterial cell wall component. NOD1 responds to γ-D-glutamyl-meso-diaminopimelic acid, which is an epitope unique to PGN structures from all Gram-negative bacteria and certain Gram-positive bacteria. Upon ligand recognition, NOD1 undergoes conformational changes and self-oligomerization mediated by the nucleotide-binding NACHT domains, followed by the recruitment and activation of the serine threonine kinase receptor-interacting protein 2 leading to the activation of NF-κB and MAPK pathways and induction of inflammatory genes. Much of our knowledge is derived from seminal studies using mice deficient in NOD1 and confirming an essential role for NOD1 in the host immune response against gastrointestinal and respiratory pathogens. In addition, recent studies have revealed a role for intracellular NOD1 receptors in the regulation of vascular inflammation and metabolism. This review will discuss our current understanding of intracellular NOD1 receptors in host immunity and chronic inflammatory disorders with a focus on cardiovascular diseases. Although therapeutic advances may have to wait until the complex interplay with pathogens, danger signals, other pattern recognition receptors and overlapping metabolic pathways is further unravelled, the steadily growing body of knowledge suggest that NOD1 antagonism might represent attractive candidate to reduce excessive inflammation associated to intestinal, cardiovascular and metabolic diseases.
Keywords: atherosclerosis; endothelium; intestinal immunity; metabolic syndrome; pulmonary immunity; receptor-interacting protein 2; septic shock.
© 2013 The British Pharmacological Society.
Figures
References
-
- Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. - PubMed
-
- Allison CC, Ferrand J, McLeod L, Hassan M, Kaparakis-Liaskos M, Grubman A, et al. Nucleotide oligomerization domain 1 enhances IFN-gamma signaling in gastric epithelial cells during Helicobacter pylori infection and exacerbates disease severity. J Immunol. 2013;190:3706–3715. - PubMed
-
- Andraws R, Berger JS, Brown DL. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2641–2647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

