Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials
- PMID: 23848435
- PMCID: PMC3729659
- DOI: 10.1186/1465-9921-14-73
Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials
Abstract
Background: Acute exacerbation of idiopathic pulmonary fibrosis has become an important outcome measure in clinical trials. This study aimed to explore the concept of suspected acute exacerbation as an outcome measure.
Methods: Three investigators retrospectively reviewed subjects enrolled in the Sildenafil Trial of Exercise Performance in IPF who experienced a respiratory serious adverse event during the course of the study. Events were classified as definite acute exacerbation, suspected acute exacerbation, or other, according to established criteria.
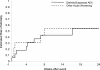
Results: Thirty-five events were identified. Four were classified as definite acute exacerbation, fourteen as suspected acute exacerbation, and seventeen as other. Definite and suspected acute exacerbations were clinically indistinguishable. Both were most common in the winter and spring months and were associated with a high risk of disease progression and short-term mortality.
Conclusions: In this study one half of respiratory serious adverse events were attributed to definite or suspected acute exacerbations. Suspected acute exacerbations are clinically indistinguishable from definite acute exacerbations and represent clinically meaningful events. Clinical trialists should consider capturing both definite and suspected acute exacerbations as outcome measures.
Figures



References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

