Measure once, cut twice--adding patient-reported outcome measures to the electronic health record for comparative effectiveness research
- PMID: 23849145
- PMCID: PMC3779680
- DOI: 10.1016/j.jclinepi.2013.04.005
Measure once, cut twice--adding patient-reported outcome measures to the electronic health record for comparative effectiveness research
Abstract
Objective: This article presents the current state of patient-reported outcome measures and explains new opportunities for leveraging the recent adoption of electronic health records to expand the application of patient-reported outcomes in both clinical care and comparative effectiveness research.
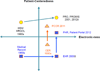
Study design and setting: Historic developments of patient-reported outcome, electronic health record, and comparative effectiveness research are analyzed in two dimensions: patient centeredness and digitization. We pose the question, "What needs to be standardized around the collection of patient-reported outcomes in electronic health records for comparative effectiveness research?"
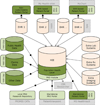
Results: We identified three converging trends: the progression of patient-reported outcomes toward greater patient centeredness and electronic adaptation; the evolution of electronic health records into personalized and fully digitized solutions; and the shift toward patient-oriented comparative effectiveness research. Related to this convergence, we propose an architecture for patient-reported outcome standardization that could serve as a first step toward a more comprehensive integration of patient-reported outcomes with electronic health record for both practice and research.
Conclusion: The science of patient-reported outcome measurement has matured sufficiently to be integrated routinely into electronic health records and other electronic health solutions to collect data on an ongoing basis for clinical care and comparative effectiveness research. Further efforts and ideally coordinated efforts from various stakeholders are needed to refine the details of the proposed framework for standardization.
Keywords: Comparative effectiveness research; Electronic health record; Health information technology; Patient centered outcomes research; Patient-report outcome; Quality of life; Survey research.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Iglehart JK. The political fight over comparative effectiveness research. Health Aff (Millwood) 2010 Oct;29(10):1757–1760. - PubMed
-
- Patel K. Health reform's tortuous route to the patient-centered outcomes research institute. Health Aff (Millwood) 2010 Oct;29(10):1777–1782. - PubMed
-
- 111th US Congress. Public Law No. 111-148: The Patient Protection and Affordable Care Act (ACA). [GPO website] [Accessed May 12, 2012];2010 Mar 23; Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
-
- Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient's perspective into drug development and communication: an ad hoc task force report of the patient-reported outcomes (PRO) harmonization group meeting at the Food and Drug Administration. Value Health. 2001 Feb;6(5):522–531. - PubMed
-
- Karnofsky D, Abelmann W, Craver L, et al. The use of nitrogen mustard in the palliative treatment of cancer. Cancer. 1948;1:634–656.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

