A model for incorporating patient and stakeholder voices in a learning health care network: Washington State's Comparative Effectiveness Research Translation Network
- PMID: 23849146
- PMCID: PMC4097950
- DOI: 10.1016/j.jclinepi.2013.04.007
A model for incorporating patient and stakeholder voices in a learning health care network: Washington State's Comparative Effectiveness Research Translation Network
Abstract
Objective: To describe the inaugural comparative effectiveness research (CER) cohort study of Washington State's Comparative Effectiveness Research Translation Network (CERTAIN), which compares invasive with noninvasive treatments for peripheral artery disease, and to focus on the patient centeredness of this cohort study by describing it within the context of a newly published conceptual framework for patient-centered outcomes research (PCOR).
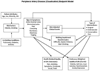
Study design and setting: The peripheral artery disease study was selected because of clinician-identified uncertainty in treatment selection and differences in desired outcomes between patients and clinicians. Patient centeredness is achieved through the "Patient Voices Project," a CERTAIN initiative through which patient-reported outcome (PRO) instruments are administered for research and clinical purposes, and a study-specific patient advisory group where patients are meaningfully engaged throughout the life cycle of the study. A clinician-led research advisory panel follows in parallel.
Results: Primary outcomes are PRO instruments that measure function, health-related quality of life, and symptoms, the latter developed with input from the patients. Input from the patient advisory group led to revised retention procedures, which now focus on short-term (3-6 months) follow-up. The research advisory panel is piloting a point-of-care, patient assessment checklist, thereby returning study results to practice. The cohort study is aligned with the tenets of one of the new conceptual frameworks for conducting PCOR.
Conclusion: The CERTAIN's inaugural cohort study may serve as a useful model for conducting PCOR and creating a learning health care network.
Keywords: Comparative effectiveness research; Patient-centered outcomes research; Patient-reported outcomes; Peripheral artery disease; Research infrastructure; Stakeholders.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The Surgical Care and Outcomes Assessment Program (SCOAP) is a Coordinated Quality Improvement Program of the Foundation for Health Care Quality. CERTAIN is a program of the University of Washington, the academic research and development partner of SCOAP. Personnel contributing to this study: Centers for Comparative and Health Systems Effectiveness (CHASE Alliance), University of Washington, Seattle, WA: David R. Flum, MD, MPH; Rafael Alfonso-Cristancho, MD, MSc, PhD; Alexander Clowes, MD; E. Beth Devine, PharmD, MBA, PhD; Todd Edwards, PhD, MA; Farhood Farjah, MD, MPH; Larry Kessler, ScD; Danielle Lavallee, PharmD, PhD; Mark Meissner, MD; Donald Patrick, PhD, MSPH; Sean D. Sullivan, PhD; Peter Tarczy-Hornoch, MD; Erik Van Eaton, MD; N. David Yanez III, PhD; Meliha Yetisgen-Yildiz, PhD, MSc; Allison Devlin, MS; Cheryl Armstrong, BSN, MPH; Mitchell Berman; Robin Boland; Daniel Capurro, MD; Rosemary Grant, BSN, CCRC, CPHQ; Marisha Hativa, MSHS; Marya Johansen; Sondra Johnson; Wendy Klamp, MPA; Sarah Lawrence, MA; Angela Lloyd, MS; Erin Machinchick; Stephanie Mallahan; Kate Nickel, MPH; Rahma Osman; Catherine Pagoaga; Ketki Patel; Robert Salazar; Rebecca Gaston Symons, MPH; Michael Tepper; Tomio Tran; Christina Yantsides; Megan Zadworny, MHA. Providence Everett Regional Medical Center, Everett, WA: Ellen Farrokhi, MD.
Figures


References
-
- Zilberberg MD. The clinical research enterprise. JAMA. 2011;305:604–605. - PubMed
-
- Conway PH, Clancy C. Comparative-effectiveness research – implications of the Federal Coordinating Council’s report. N Engl J Med. 2009;36(4):328–330. - PubMed
-
- Tunis SR, Benner J, McClellan M. Comparative effectiveness research: policy context, methods development and research infrastructure. Stat Med. 2010;29(19):1963–1976. - PubMed
-
- Abelson J, Forest PG, Eyles J, Smith P, Martin E, Vauvin FP. Deliberations about deliberative methods: issues in the design and evaluation of public participation processes. Soc Sci Med. 2003;57(2):239–251. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

