ECM-modulated cellular dynamics as a driving force for tissue morphogenesis
- PMID: 23849799
- PMCID: PMC3780589
- DOI: 10.1016/j.gde.2013.05.005
ECM-modulated cellular dynamics as a driving force for tissue morphogenesis
Abstract
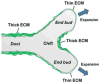
The extracellular matrix (ECM) plays diverse regulatory roles throughout development. Coordinate interactions between cells within a tissue and the ECM result in the dynamic remodeling of ECM structure. Both chemical signals and physical forces that result from such microenvironmental remodeling regulate cell behavior that sculpts tissue structure. Here, we review recent discoveries illustrating different ways in which ECM remodeling promotes dynamic cell behavior during tissue morphogenesis. We focus first on new insights that identify localized ECM signaling as a regulator of cell migration, shape, and adhesion during branching morphogenesis. We also review mechanisms by which the ECM and basement membrane can both sculpt and stabilize epithelial tissue structure, using as examples Drosophila egg chamber development and cleft formation in epithelial organs. Finally, we end with an overview of the dynamic mechanisms by which the ECM can regulate stem cell differentiation to contribute to proper tissue morphogenesis.
Published by Elsevier Ltd.
Figures



References
-
- Hynes RO, Yamada KM, editors. Extracellular matrix biology. Cold Spring Harbor Laboratory Press; 2011.
-
- Mecham RP, editor. The extracellular matrix: an overview. Springer; 2011.
-
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

