Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche
- PMID: 23850243
- PMCID: PMC3769504
- DOI: 10.1016/j.stem.2013.06.009
Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche
Abstract
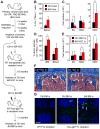
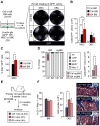
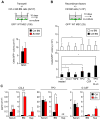
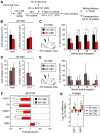
Multipotent stromal cells (MSCs) and their osteoblastic lineage cell (OBC) derivatives are part of the bone marrow (BM) niche and contribute to hematopoietic stem cell (HSC) maintenance. Here, we show that myeloproliferative neoplasia (MPN) progressively remodels the endosteal BM niche into a self-reinforcing leukemic niche that impairs normal hematopoiesis, favors leukemic stem cell (LSC) function, and contributes to BM fibrosis. We show that leukemic myeloid cells stimulate MSCs to overproduce functionally altered OBCs, which accumulate in the BM cavity as inflammatory myelofibrotic cells. We identify roles for thrombopoietin, CCL3, and direct cell-cell interactions in driving OBC expansion, and for changes in TGF-β, Notch, and inflammatory signaling in OBC remodeling. MPN-expanded OBCs, in turn, exhibit decreased expression of many HSC retention factors and severely compromised ability to maintain normal HSCs, but effectively support LSCs. Targeting this pathological interplay could represent a novel avenue for treatment of MPN-affected patients and prevention of myelofibrosis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Sinister symbiosis: pathological hematopoietic-stromal interactions in CML.Cell Stem Cell. 2013 Sep 5;13(3):257-8. doi: 10.1016/j.stem.2013.08.009. Cell Stem Cell. 2013. PMID: 24012363 Free PMC article.
References
-
- Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med. 2009;60:233–245. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
-
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. - PubMed
-
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

