Polyester modification of the mammalian TRPM8 channel protein: implications for structure and function
- PMID: 23850286
- PMCID: PMC3747310
- DOI: 10.1016/j.celrep.2013.06.022
Polyester modification of the mammalian TRPM8 channel protein: implications for structure and function
Abstract
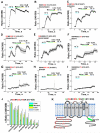
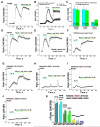
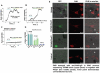
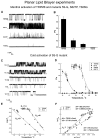
The TRPM8 ion channel is expressed in sensory neurons and is responsible for sensing environmental cues, such as cold temperatures and chemical compounds, including menthol and icilin. The channel functional activity is regulated by various physical and chemical factors and is likely to be preconditioned by its molecular composition. Our studies indicate that the TRPM8 channel forms a structural-functional complex with the polyester poly-(R)-3-hydroxybutyrate (PHB). We identified by mass spectrometry a number of PHB-modified peptides in the N terminus of the TRPM8 protein and in its extracellular S3-S4 linker. Removal of PHB by enzymatic hydrolysis and site-directed mutagenesis of both the serine residues that serve as covalent anchors for PHB and adjacent hydrophobic residues that interact with the methyl groups of the polymer resulted in significant inhibition of TRPM8 channel activity. We conclude that the TRPM8 channel undergoes posttranslational modification by PHB and that this modification is required for its normal function.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nature neuroscience. 2006;9:493–500. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt S-E, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
-
- Braaz R, Handrick R, Jendrossek D. Identification and characterisation of the catalytic triad of the alkaliphilic thermotolerant PHA depolymerase PhaZ7 of Paucimonas lemoignei. FEMS Microbiology Letters. 2003;224:107–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

