Comparison of Premier™ Rotaclone®, ProSpecT™, and RIDASCREEN® rotavirus enzyme immunoassay kits for detection of rotavirus antigen in stool specimens
- PMID: 23850415
- PMCID: PMC4602374
- DOI: 10.1016/j.jcv.2013.06.022
Comparison of Premier™ Rotaclone®, ProSpecT™, and RIDASCREEN® rotavirus enzyme immunoassay kits for detection of rotavirus antigen in stool specimens
Abstract
Background: Rotaviruses are the major cause of severe dehydrating diarrhea in children throughout the world. Enzyme immunoassays (EIAs) have been the standard method for detection of rotavirus in stool specimens since the 1980s. The World Health Organization (WHO) Rotavirus Surveillance Network has proposed including three EIA kits in the WHO-GSM (Global Management System/Système Mondial de Gestion) catalog for easy procurement of EIA kits by participating rotavirus surveillance network laboratories.
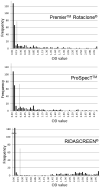
Objectives: In this study, we conducted a comparative analysis of 3 commercially available enzyme immunoassay kits: Premier™ Rotaclone® (Meridian Bioscience, Inc.), ProSpecT™ (Oxoid, Ltd.) and RIDASCREEN® (R-biopharm AG) for rotavirus diagnostics.
Study design: Using reverse-transcriptase-PCR (RT-PCR) as the gold standard, the 3 EIA kits were evaluated by testing a stool panel consisting of 56 rotavirus-positive and 54 rotavirus negative samples.
Results: The sensitivities of the Premier™ Rotaclone®, ProSpecT™ and RIDASCREEN® kits were 76.8%, 75% and 82.1%, respectively, but did not differ significantly. The specificity of all the 3 kits was 100%. The use of RT-PCR as a gold standard lowered the observed sensitivity of all 3 EIA kits but helps to reduce equivocal results that can be seen when another EIA or other non-molecular methods are used as the reference assay in comparison studies.
Conclusion: Our study found that all three kits are suitable for use by rotavirus surveillance programs.
Keywords: AGE; Comparison; EIA; ELISA; Enzyme-linked Immunosorbent Assay; FDA; Food and Drug Administration; GSM; Global Management System/Système Mondial de Gestion; IVD; NPV; OD; PPV; RT-PCR; Rotavirus; Sensitivity; Specificity; WHO; World Health Organization; acute gastroenteritis; enzyme immunoassay; in vitro diagnostic; negative predictive value; optical density; positive predictive value.
Published by Elsevier B.V.
Figures
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. - PubMed
-
- Lipson SM, Svenssen L, Goodwin L, Porti D, Danzi S, Pergolizzi R. Evaluation of two current generation enzyme immunoassays and an improved isolation-based assay for the rapid detection and isolation of rotavirus from stool. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2001;21:17–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

