Structure of N-acetyl-L-glutamate synthase/kinase from Maricaulis maris with the allosteric inhibitor L-arginine bound
- PMID: 23850694
- PMCID: PMC3754781
- DOI: 10.1016/j.bbrc.2013.07.003
Structure of N-acetyl-L-glutamate synthase/kinase from Maricaulis maris with the allosteric inhibitor L-arginine bound
Abstract
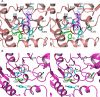
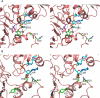
Maricaulis maris N-acetylglutamate synthase/kinase (mmNAGS/K) catalyzes the first two steps in L-arginine biosynthesis and has a high degree of sequence and structural homology to human N-acetylglutamate synthase, a regulator of the urea cycle. The synthase activity of both mmNAGS/K and human NAGS are regulated by L-arginine, although L-arginine is an allosteric inhibitor of mmNAGS/K, but an activator of human NAGS. To investigate the mechanism of allosteric inhibition of mmNAGS/K by L-arginine, we have determined the structure of the mmNAGS/K complexed with L-arginine at 2.8 Å resolution. In contrast to the structure of mmNAGS/K in the absence of L-arginine where there are conformational differences between the four subunits in the asymmetric unit, all four subunits in the L-arginine liganded structure have very similar conformations. In this conformation, the AcCoA binding site in the N-acetyltransferase (NAT) domain is blocked by a loop from the amino acid kinase (AAK) domain, as a result of a domain rotation that occurs when L-arginine binds. This structural change provides an explanation for the allosteric inhibition of mmNAGS/K and related enzymes by L-arginine. The allosterically regulated mechanism for mmNAGS/K differs significantly from that for Neisseria gonorrhoeae NAGS (ngNAGS). To define the active site, several residues near the putative active site were mutated and their activities determined. These experiments identify roles for Lys356, Arg386, Asn391 and Tyr397 in the catalytic mechanism.
Keywords: AAK; Allosteric regulation; Arginine biosynthesis; Arginine inhibition; GCN5-acetyltransferase; GCN5-related acetyltransferase; GNAT; Maricaulis maris N-acetyl-l-glutamate synthase/kinase; N-acetyl-glutamate kinase; N-acetyl-glutamate synthase; N-acetyl-l-glutamate; N-acetyl-l-glutamate kinase; N-acetyl-l-glutamate synthase; N-acetyl-l-glutamate synthase/kinase; N-acetyltransferase; NAG; NAGK; NAGS; NAGS/K; NAT; Neisseria gonorrhoeae N-acetyl-l-glutamate synthase; RMSD; Thermotoga maritima N-acetyl-l-glutamate kinase; Xanthomonas campestris N-acetyl-l-glutamate synthase/kinase; amino acid kinase; mmNAGS/K; mmNAGS/K bound with CoA; mmNAGS/K bound with l-arginine; mmNAGS/K-Arg; mmNAGS/K-CoA; ngNAGS; root mean standard deviation; tmNAGK; xcNAGS/K; yNAGK; yeast N-acetyl-l-glutamate kinase.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Ramon-Maiques S, Fernandez-Murga ML, Gil-Ortiz F, Vagin A, Fita I, Rubio V. Structural bases of feed-back control of arginine biosynthesis, revealed by the structures of two hexameric N-acetylglutamate kinases, from Thermotoga maritima and Pseudomonas aeruginosa. J Mol Biol. 2006;356:695–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

