Characterization of Neospora caninum macrophage migration inhibitory factor
- PMID: 23850997
- PMCID: PMC3805277
- DOI: 10.1016/j.exppara.2013.07.001
Characterization of Neospora caninum macrophage migration inhibitory factor
Abstract
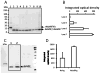
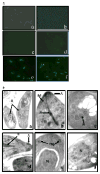
The present study is the first characterization of Neospora caninum macrophage migration inhibitory factor (NcMIF). BLAST-N analysis of NcMIF revealed high similarity (87%) to the Toxoplasma gondii MIF. NcMIF was cloned and expressed in Escherichia coli in 3 forms, NcMIF (mature protein), NcMIFm (mutation of proline-2 to glycine), and NcMIFhis (addition of a polyhistidine tag at the N-terminus). None of these recombinant NcMIFs (rNcMIF) had tautomerase, oxidoreductase, or immunologic regulatory activities. rNcMIF was unable to compete with recombinant human MIF for a MIF receptor (CD74), suggesting that NcMIF does not bind to this MIF receptor. The glycine substitution for proline-2 of NcMIF resulted in increased retention time on SEC-HPLC and decreased formation of dimers and trimers. The addition of N-terminal HIS-tag led to increased formation of trimers. Immunofluorescence staining demonstrated that NcMIF was localized to the apical end of N. caninum tachyzoites. Immunoelectron microscopy further revealed that NcMIF was present in the micronemes, rhoptries, dense granules, and nuclei. NcMIF was abundant in the tachyzoite lysate and present in excretory and secretory antigen (ESAg) preparations. Total and secretory NcMIF was more abundant in a non-pathologic clone, Ncts-8, than in the wild type isolate (NC1). Furthermore, NcMIF release by the both isolates was increased in the presence of calcium ionophore. This differential production of NcMIF by the pathologic and non-pathologic isolates of N. caninum may suggest a critical role of this molecule in the infectious pathogenesis of this parasite.
Keywords: CD74; MIF; Macrophage migration inhibitory factor; NcMIF; Neospora caninum; Tautomerase.
Published by Elsevier Inc.
Figures





Similar articles
-
Molecular characterization of a novel microneme antigen in Neospora caninum.Mol Biochem Parasitol. 2000 Apr 30;108(1):39-51. doi: 10.1016/s0166-6851(00)00200-0. Mol Biochem Parasitol. 2000. PMID: 10802317
-
Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites.Mol Biochem Parasitol. 2007 Feb;151(2):205-12. doi: 10.1016/j.molbiopara.2006.11.005. Epub 2006 Nov 30. Mol Biochem Parasitol. 2007. PMID: 17156863
-
Gene cloning and characterization of the protein encoded by the Neospora caninum bradyzoite-specific antigen gene BAG1.J Parasitol. 2013 Jun;99(3):453-8. doi: 10.1645/12-65.1. Epub 2012 Dec 17. J Parasitol. 2013. PMID: 23245337
-
Characterization of MIF family proteins: MIF and DDT from rock bream, Oplegnathus fasciatus.Fish Shellfish Immunol. 2013 Aug;35(2):458-68. doi: 10.1016/j.fsi.2013.05.003. Epub 2013 May 18. Fish Shellfish Immunol. 2013. PMID: 23688964
-
Molecular characterization of Neospora caninum MAG1, a dense granule protein secreted into the parasitophorous vacuole, and associated with the cyst wall and the cyst matrix.Parasitology. 2010 Sep;137(11):1605-19. doi: 10.1017/S0031182010000442. Epub 2010 May 6. Parasitology. 2010. PMID: 20444303
Cited by
-
Gene Expression Profiling of Neospora caninum in Bovine Macrophages Reveals Differences Between Isolates Associated With Key Parasite Functions.Front Cell Infect Microbiol. 2019 Oct 15;9:354. doi: 10.3389/fcimb.2019.00354. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31681630 Free PMC article.
-
Ostertagia ostertagi macrophage migration inhibitory factor is present in all developmental stages and may cross-regulate host functions through interaction with the host receptor.Int J Parasitol. 2014 May;44(6):355-67. doi: 10.1016/j.ijpara.2014.01.009. Epub 2014 Feb 28. Int J Parasitol. 2014. PMID: 24583184 Free PMC article.
-
Macrophage Migration Inhibitory Factor in Psoroptes ovis: Molecular Characterization and Potential Role in Eosinophil Accumulation of Skin in Rabbit and Its Implication in the Host-Parasite Interaction.Int J Mol Sci. 2023 Mar 22;24(6):5985. doi: 10.3390/ijms24065985. Int J Mol Sci. 2023. PMID: 36983058 Free PMC article.
-
Role of Host and Parasite MIF Cytokines during Leishmania Infection.Trop Med Infect Dis. 2020 Mar 20;5(1):46. doi: 10.3390/tropicalmed5010046. Trop Med Infect Dis. 2020. PMID: 32244916 Free PMC article. Review.
-
Characterization of Ostertagia ostertagi annexin-like proteins at different developmental stages.Parasitol Res. 2017 May;116(5):1515-1522. doi: 10.1007/s00436-017-5428-8. Epub 2017 Mar 29. Parasitol Res. 2017. PMID: 28378195
References
-
- Abe R, Peng T, Sailors J, Bucala R, Metz CN. Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol. 2001;166:747–753. - PubMed
-
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. - PubMed
-
- Alam A, Goyal M, Iqbal MS, Bindu S, Dey S, Pal C, Maity P, Mascarenhas NM, Ghoshal N, Bandyopadhyay U. Cysteine-3 and cysteine-4 are essential for the thioredoxin-like oxidoreductase and antioxidant activities of Plasmodium falciparum macrophage migration inhibitory factor. Free Radic Biol Med. 2011;50:1659–1668. - PubMed
-
- Arnoys EJ, Wang JL. Dual localization: proteins in extracellular and intracellular compartments. Acta Histochem. 2007;109:89–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

