Serotonin gene variants are unlikely to play a significant role in the pathogenesis of the sudden infant death syndrome
- PMID: 23851109
- PMCID: PMC3812255
- DOI: 10.1016/j.resp.2013.07.001
Serotonin gene variants are unlikely to play a significant role in the pathogenesis of the sudden infant death syndrome
Abstract
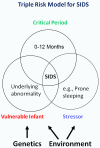
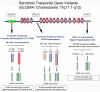
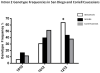
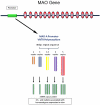
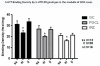
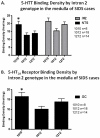
Sudden infant death syndrome (SIDS) is defined as the sudden and unexpected death of an infant less than 12 months of age that is related to a sleep period and remains unexplained after a complete autopsy, death scene investigation, and review of the clinical history. The cause of SIDS is unknown, but a major subset of SIDS is proposed to result from abnormalities in serotonin (5-HT) and related neurotransmitters in regions of the lower brainstem that result in failure of protective homeostatic responses to life-threatening challenges during sleep. Multiple studies have implicated gene variants that affect different elements of 5-HT neurotransmission in the pathogenesis of these abnormalities in SIDS. In this review I discuss the data from these studies together with some new data correlating genotype with brainstem 5-HT neurochemistry in the same SIDS cases and conclude that these gene variants are unlikely to play a major role in the pathogenesis of the medullary 5-HT abnormalities observed in SIDS.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures



References
-
- Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, Towbin JA. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. Jama. 2001;286:2264–2269. - PubMed
-
- Ambler MW, Neave C, Sturner WQ. Sudden and unexpected death in infancy and childhood: neuropathological findings. Am J Forensic Med Pathol. 1981;2:23–30. - PubMed
-
- Antila KJ, Valimaki IA, Makela M, Tuominen J, Wilson AJ, Southall DP. Heart rate variability in infants subsequently suffering sudden infant death syndrome (SIDS) Early Hum Dev. 1990;22:57–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

