Cyclosporine-inhibitable blood-brain barrier drug transport influences clinical morphine pharmacodynamics
- PMID: 23851346
- PMCID: PMC3823830
- DOI: 10.1097/ALN.0b013e3182a05bd3
Cyclosporine-inhibitable blood-brain barrier drug transport influences clinical morphine pharmacodynamics
Abstract
Background: The blood-brain barrier is richly populated by active influx and efflux transporters influencing brain drug concentrations. Morphine, a drug with delayed clinical onset, is a substrate for the efflux transporter P-glycoprotein in vitro and in animals. This investigation tested whether morphine is a transporter substrate in humans.
Methods: Fourteen healthy volunteers received morphine (0.1 mg/kg, 1-h IV infusion) in a crossover study without (control) or with the infusion of validated P-glycoprotein inhibitor cyclosporine (5 mg/kg, 2-h infusion). Plasma and urine morphine and morphine glucuronide metabolite concentrations were measured by mass spectrometry. Morphine effects were measured by miosis and analgesia.
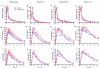
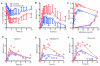
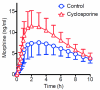
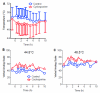
Results: Cyclosporine minimally altered morphine disposition, increasing the area under the plasma morphine concentration versus time curve to 100 ± 21 versus 85 ± 24 ng/ml·h (P < 0.05) without changing maximum plasma concentration. Cyclosporine enhanced (3.2 ± 0.9 vs. 2.5 ± 1.0 mm peak) and prolonged miosis, and increased the area under the miosis-time curve (18 ± 9 vs. 11 ± 5 mm·h), plasma effect-site transfer rate constant (k(e0), median 0.27 vs. 0.17 h(-1)), and maximum calculated effect-site morphine concentration (11.5 ± 3.7 vs. 7.6 ± 2.9 ng/ml; all P < 0.05). Analgesia testing was confounded by cyclosporine-related pain.
Conclusions: Morphine is a transporter substrate at the human blood-brain barrier. Results suggest a role for P-glycoprotein or other efflux transporters in brain morphine access, although the magnitude of the effect is small, and unlikely to be a major determinant of morphine clinical effects. Efflux may explain some variability in clinical morphine effects.
Figures

References
-
- Lotsch J. Pharmacokinetic-pharmacodynamic modeling of opioids. J Pain Symptom Manage. 2005;29:S90–103. - PubMed
-
- Tournier N, Decleves X, Saubamea B, Scherrmann JM, Cisternino S. Opioid transport by ATP-binding cassette transporters at the blood-brain barrier: Implications for neuropsychopharmacology. Curr Pharm Des. 2011;17:2829–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

