Angiogenin mediates androgen-stimulated prostate cancer growth and enables castration resistance
- PMID: 23851444
- PMCID: PMC3800479
- DOI: 10.1158/1541-7786.MCR-13-0072
Angiogenin mediates androgen-stimulated prostate cancer growth and enables castration resistance
Abstract
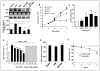
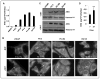
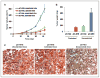
The androgen receptor (AR) is a critical effector of prostate cancer development and progression. Androgen-dependent prostate cancer is reliant on the function of AR for growth and progression. Most castration-resistant prostate cancer (CRPC) remains dependent on AR signaling for survival and growth. Ribosomal RNA (rRNA) is essential for both androgen-dependent and castration-resistant growth of prostate cancer cells. During androgen-dependent growth of prostate cells, androgen-AR signaling leads to the accumulation of rRNA. However, the mechanism by which AR regulates rRNA transcription is unknown. Here, investigation revealed that angiogenin (ANG), a member of the secreted ribonuclease superfamily, is upregulated in prostate cancer and mediates androgen-stimulated rRNA transcription in prostate cancer cells. Upon androgen stimulation, ANG undergoes nuclear translocation in androgen-dependent prostate cancer cells, where it binds to the rDNA promoter and stimulates rRNA transcription. ANG antagonists inhibit androgen-induced rRNA transcription and cell proliferation in androgen-dependent prostate cancer cells. Interestingly, ANG also mediates androgen-independent rRNA transcription through a mechanism that involves its constitutive nuclear translocation in androgen-insensitive prostate cancer cells, resulting in a constant rRNA overproduction and thereby stimulating cell proliferation. Critically, ANG overexpression in androgen-dependent prostate cancer cells enables castration-resistant growth of otherwise androgen-dependent cells. Thus, ANG-stimulated rRNA transcription is not only an essential component for androgen-dependent growth of prostate cancer but also contributes to the transition of prostate cancer from androgen-dependent to castration-resistant growth status.
Implications: The ability of angiogenin to regulate rRNA transcription and prostate cancer growth makes it a viable target for therapy.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

