The FoxO-BNIP3 axis exerts a unique regulation of mTORC1 and cell survival under energy stress
- PMID: 23851496
- PMCID: PMC4365448
- DOI: 10.1038/onc.2013.273
The FoxO-BNIP3 axis exerts a unique regulation of mTORC1 and cell survival under energy stress
Erratum in
- Oncogene. 2014 Nov 6;33(45):5310. TCGA Research Network [removed]
Abstract
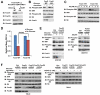
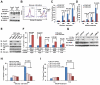
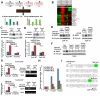
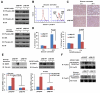
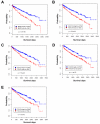
Normal cells possess adaptive mechanisms to couple energy availability with cell growth (cell size increase) and survival, and imbalances are associated with major diseases such as cancer. Inactivation of critical regulators involved in energy stress response, including adenosine monophosphate-activated protein kinase (AMPK), liver kinase B1 (LKB1), tuberous sclerosis complex 1 (TSC1) and tuberous sclerosis complex 2 (TSC2), leads to uncontrolled cell growth yet increased apoptosis under energy stress. These energy stress regulators are also important in tumor suppression and metabolism. Here, we show that forkhead box O (FoxO) transcription factor, a central regulator of tumor suppression and metabolism, plays a unique role in energy stress response. FoxOs inhibit the mammalian target of rapamycin complex 1 (mTORC1), a key regulator of cell growth, under energy stress, and inactivation of FoxOs alleviates energy stress-mediated mTORC1 repression. Surprisingly, unlike AMPK-, Lkb1- or Tsc1/2-deficient cells, FoxO-deficient cells exhibit decreased apoptosis under energy stress. FoxOs operate to inhibit mTORC1 signaling and cell survival independent of AMPK and TSC. Integrated transcriptomic and functional analyses identified BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3)-a negative regulator of both Rheb and Bcl2 prosurvival family members-as a key downstream target of FoxOs to inhibit mTORC1 function and promote apoptosis in response to energy stress. We show that p38β, but not AMPK, is likely to function upstream of FoxO-BNIP3 to mediate energy stress response. Finally, we reveal that low expression of FoxO or BNIP3 correlates with poor clinical outcomes in renal cancer patients. Together, our study uncovers a novel signaling circuit functioning to mediate cellular energy responses to control cell growth and survival. These findings also have important implications to human cancers.
Figures


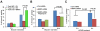

References
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006 Feb 10;124(3):471–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

