VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level
- PMID: 23851566
- PMCID: PMC3783158
- DOI: 10.1093/nar/gkt539
VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level
Abstract
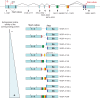
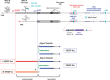
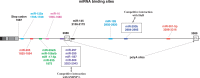
Vascular Endothelial Growth Factor A (VEGF-A) is a potent secreted mitogen crucial for physiological and pathological angiogenesis. Post-transcriptional regulation of VEGF-A occurs at multiple levels. Firstly, alternative splicing gives rise to different transcript variants encoding diverse isoforms that exhibit distinct biological properties with regard to receptor binding and extra-cellular localization. Secondly, VEGF-A mRNA stability is regulated by effectors such as hypoxia or growth factors through the binding of stabilizing and destabilizing proteins at AU-rich elements located in the 3'-untranslated region. Thirdly, translation of VEGF-A mRNA is a controlled process involving alternative initiation codons, internal ribosome entry sites (IRESs), an upstream open reading frame (uORF), miRNA targeting and a riboswitch in the 3' untranslated region. These different levels of regulation cooperate for the crucial fine-tuning of the expression of VEGF-A variants. This review will be focused on our current knowledge of the complex post-transcriptional regulatory switches that modulate the cellular VEGF-A level, a paradigmatic model of post-transcriptional regulation.
Figures
Similar articles
-
An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform.Nucleic Acids Res. 2008 Apr;36(7):2434-45. doi: 10.1093/nar/gkn093. Epub 2008 Feb 26. Nucleic Acids Res. 2008. PMID: 18304943 Free PMC article.
-
Post-transcriptional regulation of vascular endothelial growth factor: implications for tumor angiogenesis.World J Gastroenterol. 2006 Aug 21;12(31):4937-42. doi: 10.3748/wjg.v12.i31.4937. World J Gastroenterol. 2006. PMID: 16937487 Free PMC article. Review.
-
Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis.Mol Cell Biol. 2008 Jan;28(2):772-83. doi: 10.1128/MCB.02078-06. Epub 2007 Nov 26. Mol Cell Biol. 2008. PMID: 18039850 Free PMC article.
-
Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription.Oncogene. 1998 Jul 16;17(2):227-36. doi: 10.1038/sj.onc.1202019. Oncogene. 1998. PMID: 9674707
-
Non-coding RNA regulatory networks in post-transcriptional regulation of VEGFA in cancer.IUBMB Life. 2023 Jan;75(1):30-39. doi: 10.1002/iub.2620. Epub 2022 May 10. IUBMB Life. 2023. PMID: 35467790 Free PMC article. Review.
Cited by
-
Stabilization of the G-quadruplex at the VEGF IRES represses cap-independent translation.RNA Biol. 2015;12(3):320-9. doi: 10.1080/15476286.2015.1017236. RNA Biol. 2015. PMID: 25826664 Free PMC article.
-
Differential expression of angiogenesis-related miRNAs and VEGFA in cirrhosis and hepatocellular carcinoma.Arch Med Sci. 2020 Aug 10;16(5):1150-1157. doi: 10.5114/aoms.2020.97967. eCollection 2020. Arch Med Sci. 2020. PMID: 32864004 Free PMC article.
-
IL-17 in Peritoneal Dialysis-Associated Inflammation and Angiogenesis: Conclusions and Perspectives.Front Physiol. 2018 Nov 26;9:1694. doi: 10.3389/fphys.2018.01694. eCollection 2018. Front Physiol. 2018. PMID: 30534087 Free PMC article. Review.
-
Translational Control in Cancer.Cold Spring Harb Perspect Biol. 2019 Jul 1;11(7):a032896. doi: 10.1101/cshperspect.a032896. Cold Spring Harb Perspect Biol. 2019. PMID: 29959193 Free PMC article. Review.
-
More than just scanning: the importance of cap-independent mRNA translation initiation for cellular stress response and cancer.Cell Mol Life Sci. 2017 May;74(9):1659-1680. doi: 10.1007/s00018-016-2428-2. Epub 2016 Dec 2. Cell Mol Life Sci. 2017. PMID: 27913822 Free PMC article. Review.
References
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
-
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005:209–231. - PubMed
-
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl. 3):4–10. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. - PubMed
-
- Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

