Mimicking cellular transport mechanism in stem cells through endosomal escape of new peptide-coated quantum dots
- PMID: 23851637
- PMCID: PMC3711047
- DOI: 10.1038/srep02184
Mimicking cellular transport mechanism in stem cells through endosomal escape of new peptide-coated quantum dots
Abstract
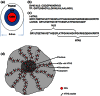
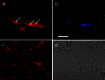
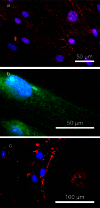
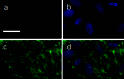
Protein transport is an important phenomenon in biological systems. Proteins are transported via several mechanisms to reach their destined compartment of cell for its complete function. One such mechanism is the microtubule mediated protein transport. Up to now, there are no reports on synthetic systems mimicking the biological protein transport mechanism. Here we report a highly efficient method of mimicking the microtubule mediated protein transport using newly designed biotinylated peptides encompassing a microtubule-associated sequence (MTAS) and a nuclear localization signaling (NLS) sequence, and their final conjugation with streptavidin-coated CdSe/ZnS quantum dots (QDs). Our results demonstrate that these novel bio-conjugated QDs enhance the endosomal escape and promote targeted delivery into the nucleus of human mesenchymal stem cells via microtubules. Mimicking the cellular transport mechanism in stem cells is highly desirable for diagnostics, targeting and therapeutic applications, opening up new avenues in the area of drug delivery.
Figures


References
-
- Gao X., Cui Y., Levenson R. M., Chung L. W. & Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004). - PubMed
-
- Medintz I. L., Uyeda H. T., Goldman E. R. & Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 4, 435–446 (2005). - PubMed
-
- Shen R. et al. Multifunctional conjugates to prepare nucleolar-targeting CdS quantum dots. J. Am. Chem. Soc. 132, 8627–8634 (2010). - PubMed
-
- Jiang W., Kim B. Y., Rutka J. T. & Chan W. C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

