Critical role of p38 and GATA3 in natural helper cell function
- PMID: 23851685
- PMCID: PMC3765427
- DOI: 10.4049/jimmunol.1300379
Critical role of p38 and GATA3 in natural helper cell function
Erratum in
- J Immunol. 2014 Aug 1;193(3):1512
Abstract
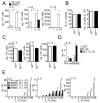
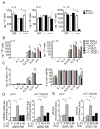
Natural helper (NH) cells, a member of Lin(-)IL-2R(+)IL-7R(+)IL-25R(+)IL-33R(+)GATA3(+) group 2 innate lymphoid cell subset, are characterized by the expression of transcription factors GATA3 and RORα and production of large amounts of Th2 cytokines such as IL-5, IL-6, and IL-13 upon IL-33 stimulation or a combination of IL-2 and IL-25. We have studied the signal transduction pathways critical for the cytokine expression and development of NH cell. Either stimulation with IL-33 or a combination of IL-2 and IL-25 induced p38 activation and phosphorylation of GATA3 in NH cells, and the phosphorylated form of GATA3 bound to the IL-5 and IL-13 promoters. All these events were blocked by SB203580, a p38 inhibitor. Inhibition of p38 also blocked IL-6 production. The mature NH cells lacking Gata3 were impaired in the proliferation and production of IL-5 and IL-13, but not IL-6, indicating that both p38 and GATA3 are critical for the proliferation and production of IL-5 and IL-13 and that the mechanisms downstream of p38 differ between IL-6 and IL-5/IL-13. In contrast, the NH cells lacking RORα showed no impairment in the proliferation and cytokine production, indicating that GATA3 but not RORα plays a pivotal role in the effector functions of mature NH cell. However, deletion of either GATA3 or RORα in hematopoietic stem cells severely blocked the development into NH cells. Our results demonstrate the important roles of p38 and GATA3 in NH cell functions.
Conflict of interest statement
S.K. is a consultant for Medical and Biological Laboratories, Co. Ltd. The authors otherwise have no financial conflicts of interest.
Figures

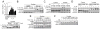


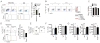
References
-
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature. 2010;463:540–544. - PubMed
-
- Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. - PubMed
-
- Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. - PubMed
-
- Koyasu S, Moro K, Tanabe M, Takeuchi T. Natural helper cells: a new player in the innate immune response against helminth infection. Adv Immunol. 2010;108:21–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

