Regulatory non-coding RNAs in pluripotent stem cells
- PMID: 23852015
- PMCID: PMC3742248
- DOI: 10.3390/ijms140714346
Regulatory non-coding RNAs in pluripotent stem cells
Abstract
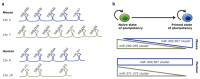
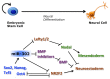
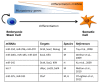
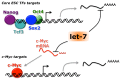
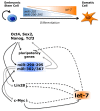
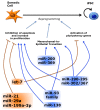
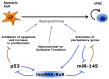
The most part of our genome encodes for RNA transcripts are never translated into proteins. These include families of RNA molecules with a regulatory function, which can be arbitrarily subdivided in short (less than 200 nucleotides) and long non-coding RNAs (ncRNAs). MicroRNAs, which act post-transcriptionally to repress the function of target mRNAs, belong to the first group. Included in the second group are multi-exonic and polyadenylated long ncRNAs (lncRNAs), localized either in the nucleus, where they can associate with chromatin remodeling complexes to regulate transcription, or in the cytoplasm, acting as post-transcriptional regulators. Pluripotent stem cells, such as embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), represent useful systems for modeling normal development and human diseases, as well as promising tools for regenerative medicine. To fully explore their potential, however, a deep understanding of the molecular basis of stemness is crucial. In recent years, increasing evidence of the importance of regulation by ncRNAs in pluripotent cells is accumulating. In this review, we will discuss recent findings pointing to multiple roles played by regulatory ncRNAs in ESC and iPSCs, where they act in concert with signaling pathways, transcriptional regulatory circuitries and epigenetic factors to modulate the balance between pluripotency and differentiation.
Figures
References
-
- James D., Levine A.J., Besser D., Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. - PubMed
-
- Vallier L., Alexander M., Pedersen R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. - PubMed
-
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

