Developmental origins of central norepinephrine neuron diversity
- PMID: 23852112
- PMCID: PMC4319358
- DOI: 10.1038/nn.3458
Developmental origins of central norepinephrine neuron diversity
Abstract
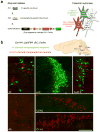
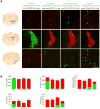
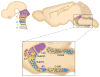
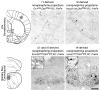
Central norepinephrine-producing neurons comprise a diverse population of cells differing in anatomical location, connectivity, function and response to disease and environmental insult. The mechanisms that generate this diversity are unknown. Here we elucidate the lineal relationship between molecularly distinct progenitor populations in the developing mouse hindbrain and mature norepinephrine neuron subtype identity. We have identified four genetically separable subpopulations of mature norepinephrine neurons differing in their anatomical location, axon morphology and efferent projection pattern. One of the subpopulations showed an unexpected projection to the prefrontal cortex, challenging the long-held belief that the locus coeruleus is the sole source of norepinephrine projections to the cortex. These findings reveal the embryonic origins of central norepinephrine neurons and provide multiple molecular points of entry for future study of individual norepinephrine circuits in complex behavioral and physiological processes including arousal, attention, mood, memory, appetite and homeostasis.
Figures



References
-
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research Brain research reviews. 2003;42:33–84. - PubMed
-
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. - PubMed
-
- Dahlström A, Fuxe K. Evidence for the existence of monoamine containing neurons in the central nervous system, I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62(Suppl. 232):1–55. - PubMed
-
- Grzanna R, Fritschy JM. Efferent projections of different subpopulations of central noradrenaline neurons. Prog Brain Res. 1991;88:89–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

