Molecular mechanisms of ATP secretion during immunogenic cell death
- PMID: 23852373
- PMCID: PMC3857631
- DOI: 10.1038/cdd.2013.75
Molecular mechanisms of ATP secretion during immunogenic cell death
Abstract
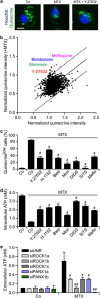
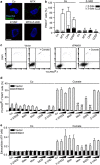
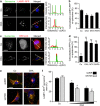
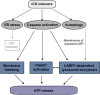
The immunogenic demise of cancer cells can be induced by various chemotherapeutics, such as anthracyclines and oxaliplatin, and provokes an immune response against tumor-associated antigens. Thus, immunogenic cell death (ICD)-inducing antineoplastic agents stimulate a tumor-specific immune response that determines the long-term success of therapy. The release of ATP from dying cells constitutes one of the three major hallmarks of ICD and occurs independently of the two others, namely, the pre-apoptotic exposure of calreticulin on the cell surface and the postmortem release of high-mobility group box 1 (HMBG1) into the extracellular space. Pre-mortem autophagy is known to be required for the ICD-associated secretion of ATP, implying that autophagy-deficient cancer cells fail to elicit therapy-relevant immune responses in vivo. However, the precise molecular mechanisms whereby ATP is actively secreted in the course of ICD remain elusive. Using a combination of pharmacological screens, silencing experiments and techniques to monitor the subcellular localization of ATP, we show here that, in response to ICD inducers, ATP redistributes from lysosomes to autolysosomes and is secreted by a mechanism that requires the lysosomal protein LAMP1, which translocates to the plasma membrane in a strictly caspase-dependent manner. The secretion of ATP additionally involves the caspase-dependent activation of Rho-associated, coiled-coil containing protein kinase 1 (ROCK1)-mediated, myosin II-dependent cellular blebbing, as well as the opening of pannexin 1 (PANX1) channels, which is also triggered by caspases. Of note, although autophagy and LAMP1 fail to influence PANX1 channel opening, PANX1 is required for the ICD-associated translocation of LAMP1 to the plasma membrane. Altogether, these findings suggest that caspase- and PANX1-dependent lysosomal exocytosis has an essential role in ATP release as triggered by immunogenic chemotherapy.
Figures




References
-
- Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. - PubMed
-
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. - PubMed
-
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

