Cell-penetrating peptide secures an efficient endosomal escape of an intact cargo upon a brief photo-induction
- PMID: 23852439
- PMCID: PMC11113630
- DOI: 10.1007/s00018-013-1416-z
Cell-penetrating peptide secures an efficient endosomal escape of an intact cargo upon a brief photo-induction
Abstract
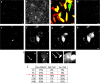
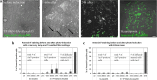
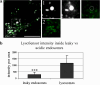
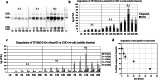
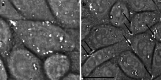
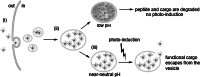
Since their discovery, cell-penetrating peptides (CPPs) have provided a novel, efficient, and non-invasive mode of transport for various (bioactive) cargos into cells. Despite the ever-growing number of successful implications of the CPP-mediated delivery, issues concerning their intracellular trafficking, significant targeting to degradative organelles, and limited endosomal escape are still hindering their widespread use. To overcome these obstacles, we have utilized a potent photo-induction technique with a fluorescently labeled protein cargo attached to an efficient CPP, TP10. In this study we have determined some key requirements behind this induced escape (e.g., dependence on peptide-to-cargo ratio, time and cargo), and have semi-quantitatively assessed the characteristics of the endosomes that become leaky upon this treatment. Furthermore, we provide evidence that the photo-released cargo remains intact and functional. Altogether, we can conclude that the photo-induced endosomes are specific large complexes-condensed non-acidic vesicles, where the released cargo remains in its native intact form. The latter was confirmed with tubulin as the cargo, which upon photo-induction was incorporated into microtubules. Because of this, we propose that combining the CPP-mediated delivery with photo-activation technique could provide a simple method for overcoming major limitations faced today and serve as a basis for enhanced delivery efficiency and a subsequent elevated cellular response of different bioactive cargo molecules.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

