RNA-based tools for nuclear reprogramming and lineage-conversion: towards clinical applications
- PMID: 23852582
- PMCID: PMC3838600
- DOI: 10.1007/s12265-013-9494-8
RNA-based tools for nuclear reprogramming and lineage-conversion: towards clinical applications
Abstract
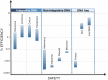
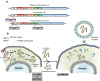
The therapeutic potential of induced pluripotent stem cells (iPSCs) is well established. Safety concerns remain, however, and these have driven considerable efforts aimed at avoiding host genome alteration during the reprogramming process. At present, the tools used to generate human iPSCs include (1) DNA-based integrative and non-integrative methods and (2) DNA-free reprogramming technologies, including RNA-based approaches. Because of their combined efficiency and safety characteristics, RNA-based methods have emerged as the most promising tool for future iPSC-based regenerative medicine applications. Here, I will discuss novel recent advances in reprogramming technology, especially those utilizing the Sendai virus (SeV) and synthetic modified mRNA. In the future, these technologies may find utility in iPSC reprogramming for cellular lineage-conversion, and its subsequent use in cell-based therapies.
Figures


References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. - PubMed
-
- Saric T, Frenzel LP, Hescheler J. Immunological barriers to embryonic stem cell-derived therapies. Cells, Tissues, Organs. 2008;188:78. - PubMed
-
- Giacomini M, Baylis F, Robert J. Banking on it: public policy and the ethics of stem cell research and development. Social Science & Medicine. 2007;65:1490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

