Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease
- PMID: 23852922
- PMCID: PMC4175400
- DOI: 10.1002/cne.23415
Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease
Abstract
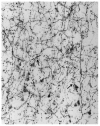
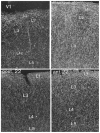
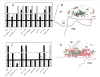
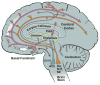
The nucleus basalis is located at the confluence of the limbic and reticular activating systems. It receives dopaminergic input from the ventral tegmental area/substantia nigra, serotonergic input from the raphe nuclei, and noradrenergic input from the nucleus locus coeruleus. Its cholinergic contingent, known as Ch4, provides the principal source of acetylcholine for the cerebral cortex and amygdala. More than half of presynaptic varicosities along its cholinergic axons make traditional synaptic contacts with cortical neurons. Limbic and paralimbic cortices of the brain receive the heaviest cholinergic input from Ch4 and are also the principal sources of reciprocal cortical projections back to the nucleus basalis. This limbic affiliation explains the role of the nucleus basalis in modulating the impact and memorability of incoming sensory information. The anatomical continuity of the nucleus basalis with other basomedial limbic structures may underlie its early and high vulnerability to the tauopathy and neurofibrillary degeneration of Alzheimer's disease. The tauopathy in Ch4 eventually leads to the degeneration of the cholinergic axons that it sends to the cerebral cortex. The early involvement of Ch4 has a magnifying effect on Alzheimer's pathology, because neurofibrillary degeneration in a small number of neurons can perturb neurotransmission in all cortical areas. Although the exact contribution of the Ch4 lesion to the cognitive changes of Alzheimer's disease remains poorly understood, the cholinergic circuitry of the nucleus basalis is emerging as one of the most strategically positioned and behaviorally consequential modulatory systems of the human cerebral cortex. J. Comp. Neurol. 521:4124-4144, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: Alzheimer's disease; cholinergic circuitry; nucleus basalis.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest Statement: The author has no conflicts of interest to disclose.
Figures









References
-
- Arendt T, Bigl V, Tennstedt A, Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer's disease. Neuroscience. 1985;14:1–14. - PubMed
-
- Aston-Jones G, Shaver R, Dinan TG. Nucleus basalis neurons exhibit axonal branching with decreased impulse conduction velocity in rat cerebrocortex. Brain Res. 1985;325:271–285. - PubMed
-
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand. 1996;165(Suppl):3–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

