Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis
- PMID: 23852992
- PMCID: PMC8078644
- DOI: 10.1002/14651858.CD007168.pub3
Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis
Abstract
Background: Cystic fibrosis is an inherited condition resulting in thickened, sticky respiratory secretions. Respiratory failure, due to recurrent pulmonary infection and inflammation, is the most common cause of mortality. Muco-active therapies (e.g. dornase alfa and nebulized hypertonic saline) may decrease sputum viscosity, increase airway clearance of sputum, reduce infection and inflammation and improve lung function. Thiol derivatives, either oral or nebulized, have shown benefit in other respiratory diseases. Their mode of action is likely to differ according to the route of administration. There are several thiol derivatives, and it is unclear which of these may be beneficial in cystic fibrosis.
Objectives: To evaluate the efficacy and safety of nebulized and oral thiol derivatives in people with cystic fibrosis.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register, comprising references identified from comprehensive electronic database searches, hand searches of relevant journals, abstract books and conference proceedings.Most recent search: 13 June 2013.We also conducted a PubMed search on 26 February 2013 for relevant published articles.
Selection criteria: Randomized and quasi-randomized controlled trials comparing nebulized or oral thiol derivatives to placebo or another thiol derivative in people with cystic fibrosis.
Data collection and analysis: The authors independently assessed trials for inclusion, analysed risk of bias and extracted data.
Main results: Searches identified 23 trials; nine trials (255 participants) are included, of these seven trials are more than 10 years old. Three trials of nebulized thiol derivatives were identified (one compared 20% N-acetylcysteine to 2% N-acetylcysteine; another compared sodium-2-mercaptoethane sulphonate to 7% hypertonic saline; and another compared glutathione to 4% hypertonic saline). Although generally well-tolerated with no significant adverse effects, there was no evidence of significant clinical benefit in our primary outcomes in participants receiving these treatments.Six trials of oral thiol derivatives were identified. Three trials compared N-acetylcysteine to placebo; one compared N-acetylcysteine, ambroxol and placebo; one compared carbocysteine to ambroxol; and one compared low and high-dose N-acetylcysteine. Oral thiol derivatives were generally well-tolerated with no significant adverse effects, however there was no evidence of significant clinical benefit in our primary outcomes in participants receiving these treatments.
Authors' conclusions: We found no evidence to recommend the use of either nebulized or oral thiol derivatives in people with cystic fibrosis. There are very few good quality trials investigating the effect of these medications in cystic fibrosis, and further research is required to investigate the potential role of these medications in improving the outcomes of people with cystic fibrosis.
Conflict of interest statement
One of the co‐authors of this review, Professor Felix Ratjen, is lead investigator on one of the included trials.
Figures



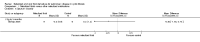
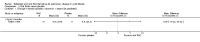
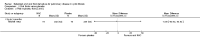
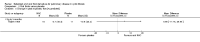
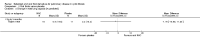
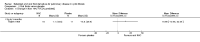

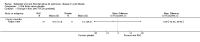
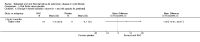


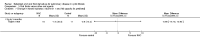
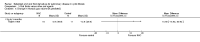

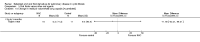
Update of
-
Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007168. doi: 10.1002/14651858.CD007168.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2013 Jul 12;(7):CD007168. doi: 10.1002/14651858.CD007168.pub3. PMID: 19160327 Updated.
References
References to studies included in this review
Bishop 2005 {published data only}
-
- Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest 2005;127(1):308‐17. - PubMed
Caramia 1995 {published data only}
-
- Caramia G, Gagliardini R, Ruffini E, Osimani P, Nobilini A. The management of cystic fibrosis with carbocysteine lysine salt: single‐blind comparative study with ambroxol hydrochloride. The Journal of International Medical Research 1995;23:284‐93. - PubMed
Dauletbaev 2009 {published data only}
Howatt 1966 {published data only}
-
- Howatt WF, DeMuth GR. A double‐blind study of the use of acetylcysteine in patients with cystic fibrosis. University of Michigan Medical Center Journal 1966;32(2):82‐5. - PubMed
Mitchell 1982 {published data only}
-
- Mitchell EA, Elliott RB. Controlled trial of oral N‐acetylcysteine in cystic fibrosis. Australian Paediatric Journal 1982;18(1):40‐2. - PubMed
Ratjen 1985 {published data only}
-
- Ratjen F, Posselt HG, WönneR, Stöver B, Bender SWJW. A double‐blind placebo controlled trial with ambroxol and N‐Acetylcysteine for mucolytic treatment in cystic fibrosis [abstract]. Proceedings of the 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:3.04.
-
- Ratjen F, Wonne R, Posselt HG, Stover B, Hofmann D, Bender SW. A double‐blind placebo controlled trial with oral ambroxol and N‐acetylcysteine for mucolytic treatment in cystic fibrosis. European Journal of Pediatrics 1985;144(4):374‐8. - PubMed
Stafanger 1988 {published data only}
-
- Stafanger G, Garne S, Howitz P, Koch C. Effect of peroral N‐acetylcysteine in patients with cystic fibrosis and primary cilia dyskinesia [abstract]. Proceedings of 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1986 Sept 1‐2; Budapest, Hungary. 1986:129.
-
- Stafanger G, Garne S, Howitz P, Morkassel E, Koch C. The clinical effect and the effect on the ciliary motility of oral N‐acetylcysteine in patients with cystic fibrosis and primary ciliary dyskinesia. European Respiratory Journal 1988;1(2):161‐7. - PubMed
Stafanger 1989 {published data only}
-
- Stafanger G, Garne S, Howitz P, Koch C. The effect of oral Nacteylcysteine on lung function CF patients [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)29.
-
- Stafanger G, Koch C. N‐acetylcysteine in cystic fibrosis and Pseudomonas aeruginosa infection: clinical score, spirometry and ciliary motility. European Respiratory Journal 1989;2(3):234‐7. - PubMed
Weller 1980 {published data only}
-
- Weller P, Ingram D, Preece M, Matthew D. A controlled trial of intermittent aerosol therapy in patients with cystic fibrosis [abstract]. Proceedings of 9th Meeting European Working Group for Cystic Fibrosis; 1979 June 12‐13; Noordwijkerhout, The Netherlands. 1979:58.
References to studies excluded from this review
App 2002 {published data only}
-
- App EM, Baran D, Dab I, Malfroot A, Coffiner M, Vanderbist F, et al. Dose‐finding and 24‐h monitoring for efficacy and safety of aerosolized Nacystelyn in cystic fibrosis. European Respiratory Journal 2002;19(2):294‐302. - PubMed
Casale 2012 {published data only}
-
- Casale A, Tosco A, Buonpensiero P, Pasqua A, Santis S, Gregorio F, et al. Inhaled GSH tolerability in patients with cystic fibrosis (CF) [abstract]. Journal of Cystic Fibrosis 2012;11 Suppl 1:S69 Abstract no: 51..
Cezeaux 1967 {published data only}
-
- Cezeaux G Jr, Telford J, Harrison G, Keats AS. Bronchial lavage in cystic fibrosis. A comparison of agents. JAMA 1967;199(1):15‐8. - PubMed
Dasgupta 1996 {published data only}
-
- Dasgupta B, King M. Reduction in viscoelasticity in cystic fibrosis sputum in vitro using combined treatment with nacystelyn and rhDNase. Pediatric Pulmonology 1996;22(3):161‐6. - PubMed
Dietzsch 1975 {published data only}
-
- Dietzsch HJ, Gottschalk B, Heyne K, Leupoid W, Wunderlich P. Cystic fibrosis: comparison of two mucolytic drugs for inhalation treatment (acetylcysteine and arginine hydrochloride). Pediatrics 1975;55(1):96‐100. - PubMed
Gotz 1980 {published data only}
-
- Gotz M, Kraemer R, Kerrebijn KF, Popow C. Oral acetylcysteine in cystic fibrosis. A co‐operative study. European Journal of Respiratory Disease. Supplement 1980;111:122‐6. - PubMed
Griese 2004 {published data only}
-
- Griese M, Ramakers J, Krasselt A, Starosta V, Koningsbruggen S, Fischer R, et al. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2004;169(7):822‐8. - PubMed
Maayan 1989 {published data only}
-
- Maayan C, Bar Yishay E, Yaacobi T, Marcus Y, Katznelson D, Yahav Y, et al. Immediate effect of various treatments on lung function in infants with cystic fibrosis. Respiration 1989;55(3):144‐51. - PubMed
Snyder 2002 {published data only}
-
- Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S‐nitrosoglutathione in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2002;165(7):922‐6. - PubMed
Tecklin 1976 {published data only}
-
- Tecklin JS, Holsclaw DS. Bronchial drainage with aerosol medications in cystic fibrosis. Physical Therapy 1976;56(9):999‐1003. - PubMed
Tirouvanziam 2006b {published data only}
-
- Tirouvanziam R, Conrad CK, Dunn CE, Davies ZA, Gernez Y, Aram B, et al. Phase 2 trial of high‐dose oral N‐Acetylcysteine as a systemic antioxidant and inhibitor of lung inflammation in CF [abstract]. Pediatric Pulmonology 2006;41(Suppl 29):295.
References to studies awaiting assessment
Griese 2013 {published data only}
-
- Griese M, Hector A, Kappler M, Ballmann M, Junge S, Rietschel E, et al. Inhaled glutathione in cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2012;11 Suppl 1:S11, Abstract no: WS5.1.
-
- Griese M, Kappler M, Eismann C, Ballmann M, Junge S, Rietschel E, et al. Inhalation treatment with glutathione in patients with cystic fibrosis: a randomised controlled trial. American Journal of Respiratory and Critical Care Medicine 2013:2013 April 30 [Epub ahead of print]. [] - PubMed
Tirouvanziam 2011 {published data only}
-
- Tirouvanziam R, Lymp J, Thompson V, Chatfield BA, Nicholls D, Clancy JP, et al. A multi‐center, phase IIB, randomized, placebo‐controlled, double‐blind study of the effects of N‐acetylcysteine (NAC) on redox changes and lung inflammation in cystic fibrosis patients [abstract]. Pediatric Pulmonology 2011;46 Suppl 34:280 Abstract no:196.
van Daele 2011 {published data only}
-
- Daele SG, Prycker S, Somville B, Meester J, Baets F. The effect of N‐acetylcysteine on the visco‐elasticity of sputum of cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2011;10 Suppl 1:S18, Abstract no: 71.
Additional references
ATS 1991
-
- American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. American Review of Respiratory Disease 1991;144(5):1202‐18. - PubMed
Bonanomi 1980
-
- Bonanomi L, Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. European Journal of Respiratory Disease 1980;111(Suppl):217‐22. - PubMed
Borregaard 1987
-
- Borregaard N, Jensen HS, Bjerrum OW. Prevention of tissue damage: inhibition of myeloperoxidase mediated inactivation of alpha 1‐proteinase inhibitor by N‐acetyl cysteine, glutathione and methionine. Agents Actions 1987;22(3‐4):255‐60. - PubMed
CFF 2007
-
- Cystic Fibrosis Foundation. Annual Report. CFF Annual Report 2007.
Corey 1996
-
- Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970‐1989. American Journal of Epidemiology 1996;143(10):1007‐17. [MEDLINE: ] - PubMed
Cotgreave 1987
-
- Cotgreave IA, Eklund A, Larsson K, Moldeus PW. No penetration of orally administered N‐acetylcysteine into bronchoalveolar lavage fluid. European Journal of Respiratory Disease 1987;70(2):73‐7. - PubMed
Curtin 2002
-
- Curtin F, Altman DG, Elbourne D. Meta‐analysis combining parallel and cross‐over clinical trials.I: Continuous outcomes. Statistics in Medicine 2002;21:2131‐44. - PubMed
Davies 2008
-
- Davies JC, Cunningham S, Alton EW, Innes JA. Lung clearance index in CF: a sensitive marker of lung disease severity. Thorax 2008;63(2):96‐97. - PubMed
Davis 2006
-
- Davis P. Cystic Fibrosis since 1938. American Journal of Respiratory and Critical Care Medicine 2006;173(5):475‐482. - PubMed
Duijvestijn 1999
-
- Duijvestijn YC, Brand PL. Systematic review of N‐acetylcysteine in cystic fibrosis. Acta Paediatrica 1999;88(1):38‐41. [MEDLINE: ] - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Elkins 2006
-
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long‐term inhaled hypertonic saline in patients with cystic fibrosis. New England Journal of Medicine 2006;354(3):229‐40. [MEDLINE: ] - PubMed
Follmann 1992
-
- Follmann D, Elliot P, Shuh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. - PubMed
Fuchs 1994
-
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. New England Journal of Medicine 1994;331(10):637‐42. [MEDLINE: ] - PubMed
Henke 2004
-
- Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. American J ournal of R espiratory C ell and M olecular B iology 2004;31(1):86‐9. - PubMed
Henke 2007
-
- Henke M, Ratjen F. Mucolytics in cystic fibrosis. Paediatric Respiratory Reviews 2007;8(1):24‐9. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 2003
Marriott 1993
-
- Marriott C, Ingham S, Coffiner M, Fossion J, Maes P. Determination of the mode of action of a novel mucolytic agent Nacystelyn. European Respiratory Journal 1993;6(Suppl 17):438s.
Rahman 2006
-
- Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal 2006;8(3‐4):681‐689. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sheffner 1964
-
- Sheffner AL, Meyer FA. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. American Review of Respiratory Disease 1964;90:721‐29. - PubMed
Shimizu 2003
-
- Shimizu T, Shimizu S, Hattori R, Gabazza EC, Majima Y. In Vivo and In Vitro Effects of Macrolide Antibiotics on Mucus Secretion in Airway Epithelial Cells. American Journal of Respiratory and Critical Care Medicine 2003;168(5):581‐7. - PubMed
Tirouvanziam 2006a
Ventresca 1989
-
- Ventresca GP, Cicchetti V, Ferrari V. Acetylcysteine. In: Braga PC, Allegra L editor(s). Drugs in Bronchial Mucology. New York: Raven Press, 1989:77‐102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

