Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2
- PMID: 23853057
- PMCID: PMC3888141
- DOI: 10.1093/jnci/djt181
Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2
Abstract
Background: Safe, effective interventions to improve cancer-related fatigue (CRF) are needed because it remains a prevalent, distressing, and activity-limiting symptom. Based on pilot data, a phase III trial was developed to evaluate the efficacy of American ginseng on CRF.
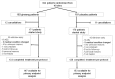
Methods: A multisite, double-blind trial randomized fatigued cancer survivors to 2000mg of American ginseng vs a placebo for 8 weeks. The primary endpoint was the general subscale of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) at 4 weeks. Changes from baseline at 4 and 8 weeks were evaluated between arms by a two-sided, two-sample t test. Toxicities were evaluated by self-report and the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) provider grading.
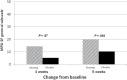
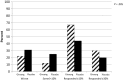
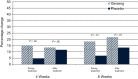
Results: Three hundred sixty-four participants were enrolled from 40 institutions. Changes from baseline in the general subscale of the MFSI-SF were 14.4 (standard deviation [SD] = 27.1) in the ginseng arm vs 8.2 (SD = 24.8) in the placebo arm at 4 weeks (P = .07). A statistically significant difference was seen at 8 weeks with a change score of 20 (SD = 27) for the ginseng group and 10.3 (SD = 26.1) for the placebo group (P = .003). Greater benefit was reported in patients receiving active cancer treatment vs those who had completed treatment. Toxicities per self-report and CTCAE grading did not differ statistically significantly between arms.
Conclusions: Data support the benefit of American ginseng, 2000mg daily, on CRF over an 8-week period. There were no discernible toxicities associated with the treatment. Studies to increase knowledge to guide the role of ginseng to improve CRF are needed.
Figures
References
-
- Arndt V, Stegmaier C, Ziegler H, et al. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107(10)2496–2503 - PubMed
-
- Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4)751–758 - PubMed
-
- Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Pych Med. 2010;40(2)163–181 - PubMed
-
- Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10 - PubMed
-
- Molassiotis A, Zheng Y, Denton-Cardew L, et al. Symptoms experienced by cancer patients during the first year from diagnosis: patient and informal caregiver ratings and agreement. Palliat Support Care. 2010;8(3)313–324 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA035267/CA/NCI NIH HHS/United States
- U10 CA060276/CA/NCI NIH HHS/United States
- CA-63844/CA/NCI NIH HHS/United States
- CA-35195/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- CA-35119/CA/NCI NIH HHS/United States
- CA-35415/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA035269/CA/NCI NIH HHS/United States
- CA-37404/CA/NCI NIH HHS/United States
- CA-35431/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- U10 CA035448/CA/NCI NIH HHS/United States
- U10 CA035195/CA/NCI NIH HHS/United States
- CA-35103/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- CA-60276/CA/NCI NIH HHS/United States
- CA-35113/CA/NCI NIH HHS/United States
- U10 CA035101/CA/NCI NIH HHS/United States
- U10 CA035415/CA/NCI NIH HHS/United States
- U10 CA063849/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- CA-37417/CA/NCI NIH HHS/United States
- CA-35269/CA/NCI NIH HHS/United States
- CA-35448/CA/NCI NIH HHS/United States
- CA-35267/CA/NCI NIH HHS/United States
- CA35101/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- CA-63849/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

