Rationale and design of the Sodium Lowering In Dialysate (SoLID) trial: a randomised controlled trial of low versus standard dialysate sodium concentration during hemodialysis for regression of left ventricular mass
- PMID: 23855560
- PMCID: PMC3720185
- DOI: 10.1186/1471-2369-14-149
Rationale and design of the Sodium Lowering In Dialysate (SoLID) trial: a randomised controlled trial of low versus standard dialysate sodium concentration during hemodialysis for regression of left ventricular mass
Abstract
Background: The current literature recognises that left ventricular hypertrophy makes a key contribution to the high rate of premature cardiovascular mortality in dialysis patients. Determining how we might intervene to ameliorate left ventricular hypertrophy in dialysis populations has become a research priority. Reducing sodium exposure through lower dialysate sodium may be a promising intervention in this regard. However there is clinical equipoise around this intervention because the benefit has not yet been demonstrated in a robust prospective clinical trial, and several observational studies have suggested sodium lowering interventions may be deleterious in some dialysis patients.
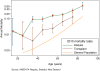
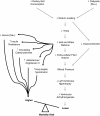
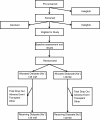
Methods/design: The Sodium Lowering in Dialysate (SoLID) study is funded by the Health Research Council of New Zealand. It is a multi-centre, prospective, randomised, single-blind (outcomes assessor), controlled parallel assignment 3-year clinical trial. The SoLID study is designed to study what impact low dialysate sodium has upon cardiovascular risk in dialysis patients. The study intends to enrol 118 home hemodialysis patients from 6 sites in New Zealand over 24 months and follow up each participant over 12 months. Key exclusion criteria are: patients who dialyse more frequently than 3.5 times per week, pre-dialysis serum sodium of <135 mM, and maintenance hemodiafiltration. In addition, some medical conditions, treatments or participation in other dialysis trials, which contraindicate the SoLID study intervention or confound its effects, will be exclusion criteria. The intervention and control groups will be dialysed using dialysate sodium 135 mM and 140 mM respectively, for 12 months. The primary outcome measure is left ventricular mass index, as measured by cardiac magnetic resonance imaging, after 12 months of intervention. Eleven or more secondary outcomes will be studied in an attempt to better understand the physiologic and clinical mechanisms by which lower dialysate sodium alters the primary end point.
Discussion: The SoLID study is designed to clarify the effect of low dialysate sodium upon the cardiovascular outcomes of dialysis patients. The study results will provide much needed information about the efficacy of a cost effective, economically sustainable solution to a condition which is curtailing the lives of so many dialysis patients.
Trial registration: Australian and New Zealand Clinical Trials Registry number: ACTRN12611000975998.
Figures
Similar articles
-
Rationale and design of the Myocardial Microinjury and Cardiac Remodeling Extension Study in the Sodium Lowering in Dialysate trial (Mac-SoLID study).BMC Nephrol. 2014 Jul 21;15:120. doi: 10.1186/1471-2369-15-120. BMC Nephrol. 2014. PMID: 25047825 Free PMC article. Clinical Trial.
-
Effect of Low-Sodium versus Conventional Sodium Dialysate on Left Ventricular Mass in Home and Self-Care Satellite Facility Hemodialysis Patients: A Randomized Clinical Trial.J Am Soc Nephrol. 2020 May;31(5):1078-1091. doi: 10.1681/ASN.2019090877. Epub 2020 Mar 18. J Am Soc Nephrol. 2020. PMID: 32188697 Free PMC article. Clinical Trial.
-
Rationale and design of a multi-centre randomised controlled trial of individualised cooled dialysate to prevent left ventricular systolic dysfunction in haemodialysis patients.BMC Nephrol. 2012 Jun 21;13:45. doi: 10.1186/1471-2369-13-45. BMC Nephrol. 2012. PMID: 22720738 Free PMC article. Clinical Trial.
-
Dialysate Sodium: Rationale for Evolution over Time.Semin Dial. 2017 Mar;30(2):99-111. doi: 10.1111/sdi.12570. Epub 2017 Jan 8. Semin Dial. 2017. PMID: 28066913 Free PMC article. Review.
-
Intensive Hemodialysis, Left Ventricular Hypertrophy, and Cardiovascular Disease.Am J Kidney Dis. 2016 Nov;68(5S1):S5-S14. doi: 10.1053/j.ajkd.2016.05.025. Am J Kidney Dis. 2016. PMID: 27772643 Review.
Cited by
-
Executive summary of the Korean Society of Nephrology 2021 clinical practice guideline for optimal hemodialysis treatment.Korean J Intern Med. 2022 Jul;37(4):701-718. doi: 10.3904/kjim.2021.543. Epub 2022 Jun 3. Korean J Intern Med. 2022. PMID: 35811360 Free PMC article.
-
Hypertension in hemodialysis patients: an opinion-based update.Semin Dial. 2014 Mar;27(2):146-53. doi: 10.1111/sdi.12195. Epub 2014 Feb 5. Semin Dial. 2014. PMID: 24494716 Free PMC article. Review.
-
Effects of dialysate to serum sodium (Na+) alignment in chronic hemodialysis (HD) patients: retrospective cohort study from a quality improvement project.BMC Nephrol. 2018 Apr 2;19(1):75. doi: 10.1186/s12882-018-0870-0. BMC Nephrol. 2018. PMID: 29609536 Free PMC article.
-
Korean Society of Nephrology 2021 Clinical Practice Guideline for Optimal Hemodialysis Treatment.Kidney Res Clin Pract. 2021 Dec;40(Suppl 1):S1-S37. doi: 10.23876/j.krcp.21.600. Epub 2021 Dec 10. Kidney Res Clin Pract. 2021. PMID: 34923803 Free PMC article. No abstract available.
-
Dietary Sodium and Other Nutrient Intakes among Patients Undergoing Hemodialysis in New Zealand.Nutrients. 2018 Apr 18;10(4):502. doi: 10.3390/nu10040502. Nutrients. 2018. PMID: 29670030 Free PMC article.
References
-
- U.S. Renal Data System, USRDS 2012 Annual Data Report. Atlas of End-stage renal disease in the united states, national institutes of health, national institute of diabetes and digestive and kidney diseases. Bethesda, MD; 2012.
-
- McDonald S. ANZDATA Registry Report, Australia and New Zealand Dialysis and Transplant Registry, Adelaide, South Australia. 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

