Virus replicon particle based Chikungunya virus neutralization assay using Gaussia luciferase as readout
- PMID: 23855906
- PMCID: PMC3718613
- DOI: 10.1186/1743-422X-10-235
Virus replicon particle based Chikungunya virus neutralization assay using Gaussia luciferase as readout
Abstract
Background: Chikungunya virus (CHIKV) has been responsible for large epidemic outbreaks causing fever, headache, rash and severe arthralgia. So far, no specific treatment or vaccine is available. As nucleic acid amplification can only be used during the viremic phase of the disease, serological tests like neutralization assays are necessary for CHIKV diagnosis and for determination of the immune status of a patient. Furthermore, neutralization assays represent a useful tool to validate the efficacy of potential vaccines. As CHIKV is a BSL3 agent, neutralization assays with infectious virus need to be performed under BSL3 conditions. Our aim was to develop a neutralization assay based on non-infectious virus replicon particles (VRPs).
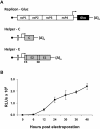
Methods: VRPs were produced by cotransfecting baby hamster kidney-21 cells with a CHIKV replicon expressing Gaussia luciferase (Gluc) and two helper RNAs expressing the CHIKV capsid protein or the remaining structural proteins, respectively. The resulting single round infectious particles were used in CHIKV neutralization assays using secreted Gluc as readout.
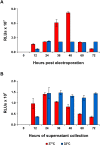
Results: Upon cotransfection of a CHIKV replicon expressing Gluc and the helper RNAs VRPs could be produced efficiently under optimized conditions at 32°C. Infection with VRPs could be measured via Gluc secreted into the supernatant. The successful use of VRPs in CHIKV neutralization assays was demonstrated using a CHIKV neutralizing monoclonal antibody or sera from CHIKV infected patients. Comparison of VRP based neutralization assays in 24- versus 96-well format using different amounts of VRPs revealed that in the 96-well format a high multiplicity of infection is favored, while in the 24-well format reliable results are also obtained using lower infection rates. Comparison of different readout times revealed that evaluation of the neutralization assay is already possible at the same day of infection.
Conclusions: A VRP based CHIKV neutralization assay using Gluc as readout represents a fast and useful method to determine CHIKV neutralizing antibodies without the need of using infectious CHIKV.
Figures
Similar articles
-
Development of a Novel Chikungunya Virus-Like Replicon Particle for Rapid Quantification and Screening of Neutralizing Antibodies and Antivirals.Microbiol Spectr. 2023 Mar 1;11(2):e0485422. doi: 10.1128/spectrum.04854-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36856407 Free PMC article.
-
High-Throughput Platform for Detection of Neutralizing Antibodies Using Flavivirus Reporter Replicon Particles.Viruses. 2022 Feb 8;14(2):346. doi: 10.3390/v14020346. Viruses. 2022. PMID: 35215941 Free PMC article.
-
Facile quantitative diagnostic testing for neutralizing antibodies against Chikungunya virus.BMC Infect Dis. 2024 Sep 30;24(1):1076. doi: 10.1186/s12879-024-09973-y. BMC Infect Dis. 2024. PMID: 39350079 Free PMC article.
-
Luciferase Immunosorbent Assay Based on Multiple E Antigens for the Detection of Chikungunya Virus-Specific IgG Antibodies.Microbiol Spectr. 2022 Apr 27;10(2):e0149621. doi: 10.1128/spectrum.01496-21. Epub 2022 Mar 21. Microbiol Spectr. 2022. PMID: 35311573 Free PMC article. Review.
-
Development of Vaccines for Chikungunya Fever.J Infect Dis. 2016 Dec 15;214(suppl 5):S488-S496. doi: 10.1093/infdis/jiw271. J Infect Dis. 2016. PMID: 27920179 Free PMC article. Review.
Cited by
-
Development of a Novel Chikungunya Virus-Like Replicon Particle for Rapid Quantification and Screening of Neutralizing Antibodies and Antivirals.Microbiol Spectr. 2023 Mar 1;11(2):e0485422. doi: 10.1128/spectrum.04854-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36856407 Free PMC article.
-
Preparation and application of chikungunya pseudovirus containing double reporter genes.Sci Rep. 2022 Jun 14;12(1):9844. doi: 10.1038/s41598-022-13230-0. Sci Rep. 2022. PMID: 35701460 Free PMC article.
-
A human genome-wide loss-of-function screen identifies effective chikungunya antiviral drugs.Nat Commun. 2016 May 12;7:11320. doi: 10.1038/ncomms11320. Nat Commun. 2016. PMID: 27177310 Free PMC article.
-
Expression of Alphavirus Nonstructural Protein 2 (nsP2) in Mosquito Cells Inhibits Viral RNA Replication in Both a Protease Activity-Dependent and -Independent Manner.Viruses. 2022 Jun 17;14(6):1327. doi: 10.3390/v14061327. Viruses. 2022. PMID: 35746799 Free PMC article.
-
Immunogenic recombinant Mayaro virus-like particles present natively assembled glycoprotein.NPJ Vaccines. 2024 Dec 17;9(1):243. doi: 10.1038/s41541-024-01021-9. NPJ Vaccines. 2024. PMID: 39690153 Free PMC article.
References
-
- Kuhn RJ. In: Fields Virology. 5. Knipe DM, Howley PM, editor. Vol. 10. Philadelphia: Lippincott Williams & Wilkons; 2007. Togaviridae: the viruses and their replicatioon; pp. 1001–1022.
-
- Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;10:389–401. doi: 10.1006/viro.1997.8878. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

