PTK6 activation at the membrane regulates epithelial-mesenchymal transition in prostate cancer
- PMID: 23856248
- PMCID: PMC3766391
- DOI: 10.1158/0008-5472.CAN-13-0443
PTK6 activation at the membrane regulates epithelial-mesenchymal transition in prostate cancer
Erratum in
- Cancer Res. 2013 Oct 1;73(19):6096
Abstract
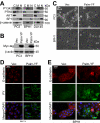
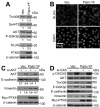
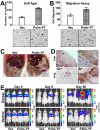
The intracellular tyrosine kinase protein tyrosine kinase 6 (PTK6) lacks a membrane-targeting SH4 domain and localizes to the nuclei of normal prostate epithelial cells. However, PTK6 translocates from the nucleus to the cytoplasm in human prostate tumor cells. Here, we show that while PTK6 is located primarily within the cytoplasm, the pool of active PTK6 in prostate cancer cells localizes to membranes. Ectopic expression of membrane-targeted active PTK6 promoted epithelial-mesenchymal transition in part by enhancing activation of AKT, thereby stimulating cancer cell migration and metastases in xenograft models of prostate cancer. Conversely, siRNA-mediated silencing of endogenous PTK6 promoted an epithelial phenotype and impaired tumor xenograft growth. In mice, PTEN deficiency caused endogenous active PTK6 to localize at membranes in association with decreased E-cadherin expression. Active PTK6 was detected at membranes in some high-grade human prostate tumors, and PTK6 and E-cadherin expression levels were inversely correlated in human prostate cancers. In addition, high levels of PTK6 expression predicted poor prognosis in patients with prostate cancer. Our findings reveal novel functions for PTK6 in the pathophysiology of prostate cancer, and they define this kinase as a candidate therapeutic target. Cancer Res; 73(17); 5426-37. ©2013 AACR.
Figures




References
-
- ACS . Cancer Facts & Figures 2013. American Cancer Society; Atlanta: 2013.
-
- Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, et al. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. Journal of cell science. 2009;122:965–75. - PubMed
-
- Ie Kim H, Lee ST. Oncogenic functions of PTK6 are enhanced by its targeting to plasma membrane but abolished by its targeting to nucleus. Journal of biochemistry. 2009;146:133–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

