Phospholamban knockout breaks arrhythmogenic Ca²⁺ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice
- PMID: 23856523
- PMCID: PMC3864692
- DOI: 10.1161/CIRCRESAHA.113.301678
Phospholamban knockout breaks arrhythmogenic Ca²⁺ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice
Abstract
Rationale: Phospholamban (PLN) is an inhibitor of cardiac sarco(endo)plasmic reticulum Ca²⁺ ATPase. PLN knockout (PLN-KO) enhances sarcoplasmic reticulum Ca²⁺ load and Ca²⁺ leak. Conversely, PLN-KO accelerates Ca²⁺ sequestration and aborts arrhythmogenic spontaneous Ca²⁺ waves (SCWs). An important question is whether these seemingly paradoxical effects of PLN-KO exacerbate or protect against Ca²⁺-triggered arrhythmias.
Objective: We investigate the impact of PLN-KO on SCWs, triggered activities, and stress-induced ventricular tachyarrhythmias (VTs) in a mouse model of cardiac ryanodine-receptor (RyR2)-linked catecholaminergic polymorphic VT.
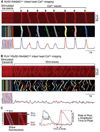
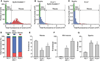
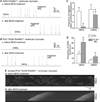
Methods and results: We generated a PLN-deficient, RyR2-mutant mouse model (PLN-/-/RyR2-R4496C+/-) by crossbreeding PLN-KO mice with catecholaminergic polymorphic VT-associated RyR2-R4496C mutant mice. Ca²⁺ imaging and patch-clamp recording revealed cell-wide propagating SCWs and triggered activities in RyR2-R4496C+/- ventricular myocytes during sarcoplasmic reticulum Ca²⁺ overload. PLN-KO fragmented these cell-wide SCWs into mini-waves and Ca²⁺ sparks and suppressed the triggered activities evoked by sarcoplasmic reticulum Ca²⁺ overload. Importantly, these effects of PLN-KO were reverted by partially inhibiting sarco(endo)plasmic reticulum Ca²⁺ ATPase with 2,5-di-tert-butylhydroquinone. However, Bay K, caffeine, or Li⁺ failed to convert mini-waves to cell-wide SCWs in PLN-/-/RyR2-R4496C+/- ventricular myocytes. Furthermore, ECG analysis showed that PLN-KO mice are not susceptible to stress-induced VTs. On the contrary, PLN-KO protected RyR2-R4496C mutant mice from stress-induced VTs.
Conclusions: Our results demonstrate that despite severe sarcoplasmic reticulum Ca²⁺ leak, PLN-KO suppresses triggered activities and stress-induced VTs in a mouse model of catecholaminergic polymorphic VT. These data suggest that breaking up cell-wide propagating SCWs by enhancing Ca²⁺ sequestration represents an effective approach for suppressing Ca²⁺-triggered arrhythmias.
Keywords: Ca2+ leak; Ca2+ waves; Ca2+-triggered arrhythmias; phospholamban; ryanodine receptor calcium release channel; sarcoplasmic reticulum.
Figures





References
-
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

