Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial
- PMID: 23856681
- PMCID: PMC3713531
- DOI: 10.7326/0003-4819-159-2-201307160-00002
Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial
Abstract
Background: Recent evidence suggests that daily aspirin use decreases cancer risk, particularly for colorectal cancer, but evidence for alternate-day use is scant.
Objective: To examine the association between long-term, alternate-day, low-dose aspirin and cancer in healthy women.
Design: Observational follow-up of a randomized trial.
Setting: Female health professionals.
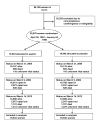
Participants: 39,876 women aged 45 years or older in the Women's Health Study (ClinicalTrials.gov: NCT00000479), 33 682 of whom continued observational follow-up.
Intervention: 100 mg of alternate-day aspirin or placebo through March 2004, with a median 10-year follow-up. Posttrial follow-up continued through March 2012.
Measurements: Cancer incidence.
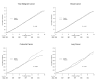
Results: A total of 5071 cancer cases (including 2070 breast, 451 colorectal, and 431 lung cancer cases) and 1391 cancer deaths were confirmed. Over the entire follow-up, aspirin had no association with total (hazard ratio [HR], 0.97 [95% CI, 0.92 to 1.03]; P = 0.31), breast (HR, 0.98 [CI, 0.90 to 1.07]; P = 0.65), or lung (HR, 1.04 [CI, 0.86 to 1.26]; P = 0.67) cancer. Colorectal cancer was reduced in the aspirin group (HR, 0.80 [CI, 0.67 to 0.97]; P = 0.021), primarily for proximal colon cancer (HR, 0.73 [CI, 0.55 to 0.95]; P = 0.022). The difference emerged after 10 years, with a posttrial reduction of 42% (HR, 0.58 [CI, 0.42 to 0.80]; P < 0.001). There was no extended effect on cancer deaths or colorectal polyps. More gastrointestinal bleeding (HR, 1.14 [CI, 1.06 to 1.22]; P < 0.001) and peptic ulcers (HR, 1.17 [CI, 1.09 to 1.27]; P < 0.001) occurred in the aspirin group.
Limitations: Not all women received extended follow-up, and posttrial ascertainment bias cannot be ruled out. Gastrointestinal bleeding, peptic ulcers, and polyps were self-reported during extended follow-up.
Conclusion: Long-term use of alternate-day, low-dose aspirin may reduce risk for colorectal cancer in healthy women.
Figures

Comment in
-
Alternate-day, low-dose aspirin and cancer risk.Ann Intern Med. 2013 Jul 16;159(2):148-50. doi: 10.7326/0003-4819-159-2-201307160-00013. Ann Intern Med. 2013. PMID: 23856684 No abstract available.
References
-
- Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–7. - PubMed
-
- Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23(6):1403–15. - PubMed
-
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
