Antiviral effect, safety, and pharmacokinetics of five-day oral administration of Deleobuvir (BI 207127), an investigational hepatitis C virus RNA polymerase inhibitor, in patients with chronic hepatitis C
- PMID: 23856779
- PMCID: PMC3811456
- DOI: 10.1128/AAC.00565-13
Antiviral effect, safety, and pharmacokinetics of five-day oral administration of Deleobuvir (BI 207127), an investigational hepatitis C virus RNA polymerase inhibitor, in patients with chronic hepatitis C
Abstract
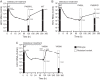
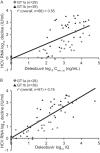
Deleobuvir (BI 207127) is an investigational oral nonnucleoside inhibitor of hepatitis C virus (HCV) NS5B RNA polymerase. Antiviral activity, virology, pharmacokinetics, and safety were assessed in HCV genotype 1-infected patients receiving 5 days' deleobuvir monotherapy. In this double-blind phase 1b study, treatment-naive (TN; n = 15) and treatment-experienced (TE; n = 45) patients without cirrhosis received placebo or deleobuvir at 100, 200, 400, 800, or 1,200 mg every 8 h (q8h) for 5 days. Patients with cirrhosis (n = 13) received deleobuvir at 400 or 600 mg q8h for 5 days. Virologic analyses included NS5B genotyping and phenotyping of individual isolates. At day 5, patients without cirrhosis had dose-dependent median HCV RNA reductions of up to 3.8 log10 (with no placebo response); patients with cirrhosis had median HCV RNA reductions of approximately 3.0 log10. Three patients discontinued due to adverse events (AEs). The most common AEs were gastrointestinal, nervous system, and skin/cutaneous tissue disorders. Plasma exposure of deleobuvir was supraproportional at doses ≥ 400 mg q8h and approximately 2-fold higher in patients with cirrhosis than in patients without cirrhosis. No virologic breakthrough was observed. NS5B substitutions associated with deleobuvir resistance in vitro were detected in 9/59 patients; seven encoded P495 substitutions, including P495L, which conferred 120- to 310-fold-decreased sensitivity to deleobuvir. P495 variants did not persist in follow-up without selective drug pressure. Deleobuvir monotherapy was generally well tolerated and demonstrated dose-dependent antiviral activity against HCV genotype 1 over 5 days.
Figures

References
-
- European Association for the Study of the Liver 2011. EASL clinical practice guidelines: management of hepatitis C virus infection. J. Hepatol. 55:245–264 - PubMed
-
- European Medicines Agency 2013. INCIVO™ (telaprevir). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... Accessed 21 June 2013
-
- Vertex Pharmaceuticals Incorporated 2013. INCIVEK™ (telaprevir). Highlights of prescribing information. http://pi.vrtx.com/files/uspi_telaprevir.pdf Accessed 21 June 2013
-
- Merck & Co 2013. VICTRELIS™ (boceprevir). Highlights of prescribing information. http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf Accessed 21 June 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

