Mucins in pancreatic cancer and its microenvironment
- PMID: 23856888
- PMCID: PMC3934431
- DOI: 10.1038/nrgastro.2013.120
Mucins in pancreatic cancer and its microenvironment
Abstract
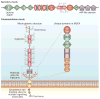
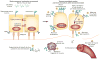
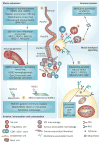
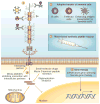
Pancreatic cancer remains a lethal malignancy with poor prognosis owing to therapeutic resistance, frequent recurrence and the absence of treatment strategies that specifically target the tumour and its supporting stroma. Deregulated cell-surface proteins drive neoplastic transformations and are envisioned to mediate crosstalk between the tumour and its microenvironment. Emerging studies have elaborated on the role of mucins in diverse biological functions, including enhanced tumorigenicity, invasiveness, metastasis and drug resistance through their characteristic O-linked and N-linked oligosaccharides (glycans), extended structures and unique domains. Multiple mucin domains differentially interact and regulate different components of the tumour microenvironment. This Review discusses: the expression pattern of various mucins in the pancreas under healthy, inflammatory, and cancerous conditions; the context-dependent attributes of mucins that differ under healthy and pathological conditions; the contribution of the tumour microenvironment in pancreatic cancer development and/or progression; diagnostic and/or prognostic efficacy of mucins; and mucin-based therapeutic strategies. Overall, this information should help to delineate the intricacies of pancreatic cancer by exploring the family of mucins, which, through various mechanisms in both tumour cells and the microenvironment, worsen disease outcome.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Cancer Facts and Statistics. American Cancer Society; 2013. online http://www.cancer.org/research/cancerfactsstatistics/
-
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. - PubMed
-
- Pflugfelder SC, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–1326. - PubMed
-
- Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

