Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study
- PMID: 23857305
- PMCID: PMC4296226
- DOI: 10.1002/hep.26617
Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study
Abstract
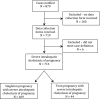
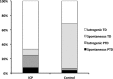
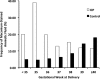
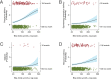
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver disease, characterized by maternal pruritus and raised serum bile acids. Our objectives were to describe the epidemiology and pregnancy complications associated with severe ICP and to test the hypothesis that adverse perinatal outcomes are increased in these women. A prospective population-based case-control study with national coverage was undertaken using the UK Obstetric Surveillance System (UKOSS). Control data for comparison were obtained from women with healthy pregnancy outcome through UKOSS (n = 2,232), St Mary's Maternity Information System (n = 554,319), and Office for National Statistics (n = 668,195). The main outcome measures investigated were preterm delivery, stillbirth, and neonatal unit admission. In all, 713 confirmed cases of severe ICP were identified, giving an estimated incidence of 9.2 per 10,000 maternities. Women with severe ICP and a singleton pregnancy (n = 669) had increased risks of preterm delivery (164/664; 25% versus 144/2200; 6.5%; adjusted odds ratio [OR] 5.39, 95% confidence interval [CI] 4.17 to 6.98), neonatal unit admission (80/654; 12% versus 123/2192; 5.6%; adjusted OR 2.68, 95% CI 1.97 to 3.65), and stillbirth (10/664; 1.5% versus 11/2205; 0.5%; adjusted OR 2.58, 95% CI 1.03 to 6.49) compared to controls. Seven of 10 stillbirths in ICP cases were associated with coexisting pregnancy complications. These differences remained significant against national data. Risks of preterm delivery, meconium-stained amniotic fluid, and stillbirth rose with increasing maternal serum bile acid concentrations.
Conclusion: In the largest prospective cohort study in severe ICP to date, we demonstrate significant increased risks of adverse perinatal outcomes, including stillbirth. Our findings support the case for close antenatal monitoring of pregnancies affected by severe ICP.
© 2014 The Authors. Hepatology published by Wiley on behalf of the American Association for the Study of Liver Diseases.
Figures
Comment in
-
Serum bile acids in intrahepatic cholestasis of pregnancy: not just a diagnostic test.Hepatology. 2014 Apr;59(4):1220-2. doi: 10.1002/hep.26888. Epub 2014 Feb 18. Hepatology. 2014. PMID: 24123247 Free PMC article. No abstract available.
References
-
- RCOG. Obstetric cholestasis. Green Top Guideline. 2011;43 No.
-
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–474. - PubMed
-
- Laatikainen T, Ikonen E. Fetal prognosis in obstetric hepatosis. Ann Chir Gynaecol Fenn. 1975;64:155–164. - PubMed
-
- Laatikainen T, Ikonen E. Serum bile acids in cholestasis of pregnancy. Obstet Gynecol. 1977;50:313–318. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
