Vitamin D 2 interacts with Human PrP(c) (90-231) and breaks PrP(c) oligomerization in vitro
- PMID: 23857314
- PMCID: PMC3904317
- DOI: 10.4161/pri.25739
Vitamin D 2 interacts with Human PrP(c) (90-231) and breaks PrP(c) oligomerization in vitro
Abstract
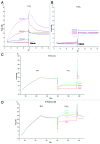
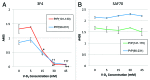
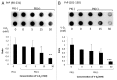
PrP(sc), the pathogenic isoform of PrP(c), can convert PrP(c) into PrP(sc) through direct interactions. PrP(c) oligomerization is a required processing step before PrP(sc) formation, and soluble oligomers appear to be the toxic species in amyloid-related disorders. In the current study, direct interactions between vitamin D 2 and human recombinant PrP(c) (90-231) were observed by Biacore assay, and 3F4 antibody, specific for amino acid fragment 109-112 of PrP(c), inhibited this interaction. An ELISA study using3F4 antibody showed that PrP(c) (101-130), corresponding sequence to human PrP, was affected by vitamin D 2, supporting the results of Biacore studies and suggesting that the PrP(c) sequence around the 3F4 epitope was responsible for the interaction with vitamin D 2. Furthermore, the effects of vitamin D 2 on disruption of PrP(c) (90-231) oligomerization were elucidated by dot blot analysis and differential protease k susceptibilities. While many chemical compounds have been proposed as potential therapeutic agents for the treatment of scrapie, most of these are toxic. However, given the safety and blood brain barrier permeability of vitamin D 2, we propose that vitamin D 2 may be a suitable agent to target PrP(c) in the brain and therefore is a potential therapeutic candidate for prion disease.
Keywords: PrPc; PrPsc; oligomerization; prion disease; vitamin D2.
Figures
Similar articles
-
Prion infection: seeded fibrillization or more?Prion. 2008 Apr-Jun;2(2):67-72. doi: 10.4161/pri.2.2.7060. Epub 2008 Apr 23. Prion. 2008. PMID: 19098436 Free PMC article. Review.
-
Formation of soluble oligomers and amyloid fibrils with physical properties of the scrapie isoform of the prion protein from the C-terminal domain of recombinant murine prion protein mPrP-(121-231).J Biol Chem. 2006 Sep 8;281(36):26121-8. doi: 10.1074/jbc.M605367200. Epub 2006 Jul 13. J Biol Chem. 2006. PMID: 16844683
-
Detection of prion epitopes on PrP and PrP of transmissible spongiform encephalopathies using specific monoclonal antibodies to PrP.Immunol Cell Biol. 2005 Dec;83(6):632-7. doi: 10.1111/j.1440-1711.2005.01384.x. Immunol Cell Biol. 2005. PMID: 16266315
-
Molecular interaction between prion protein and GFAP both in native and recombinant forms in vitro.Med Microbiol Immunol. 2008 Dec;197(4):361-8. doi: 10.1007/s00430-007-0071-0. Epub 2007 Dec 18. Med Microbiol Immunol. 2008. PMID: 18087720
-
Molecular Mechanism of the Misfolding and Oligomerization of the Prion Protein: Current Understanding and Its Implications.Biochemistry. 2015 Jul 28;54(29):4431-42. doi: 10.1021/acs.biochem.5b00605. Epub 2015 Jul 17. Biochemistry. 2015. PMID: 26171558 Review.
Cited by
-
Association of 25-hydroxyvitamin D status with brain volume changes.Food Sci Nutr. 2021 Jun 15;9(8):4169-4175. doi: 10.1002/fsn3.2382. eCollection 2021 Aug. Food Sci Nutr. 2021. PMID: 34401068 Free PMC article.
-
Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats.J Neuroinflammation. 2018 Aug 31;15(1):249. doi: 10.1186/s12974-018-1266-6. J Neuroinflammation. 2018. PMID: 30170624 Free PMC article.
-
The Role of Vitamins in Neurodegenerative Disease: An Update.Biomedicines. 2021 Sep 22;9(10):1284. doi: 10.3390/biomedicines9101284. Biomedicines. 2021. PMID: 34680401 Free PMC article. Review.
References
-
- Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
