Cost effectiveness of romiplostim for the treatment of chronic immune thrombocytopenia in Ireland
- PMID: 23857462
- PMCID: PMC3824633
- DOI: 10.1007/s40258-013-0044-y
Cost effectiveness of romiplostim for the treatment of chronic immune thrombocytopenia in Ireland
Erratum in
- Appl Health Econ Health Policy. 2013 Dec;11(6):687
Abstract
Background: Romiplostim, a thrombopoietin receptor agonist (TPOra), is a second-line medical treatment option for adults with chronic immune thrombocytopenia (ITP). Clinical trials have shown that romiplostim increases platelet counts, while reducing the risk of bleeding and, in turn, the need for costly rescue medications.
Aims: The objective of this study was to assess the cost effectiveness of romiplostim in the treatment of adult ITP in Ireland, in comparison with eltrombopag and the medical standard of care (SoC).
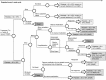
Methods: A lifetime treatment-sequence cost-utility Markov model with embedded decision tree was developed from an Irish healthcare perspective to compare romiplostim with eltrombopag and SoC. The model was driven by platelet response (platelet count ≥50 × 10(9)/L), which determined effectiveness and progression along the treatment pathway, need for rescue therapy (e.g. intravenous immunoglobulin [IVIg] and steroids) and risk of bleeding. Probability of response, mean treatment duration, average time to initial response and utilities were derived from clinical trials and other published evidence. Treatment sequences and healthcare utilization practice were validated by Irish clinical experts. Costs were assessed in <euro> for 2011 and included drug acquisition costs and costs associated with monitoring patients and management of bleeding, as available from published Irish reimbursement lists and other relevant sources. Deterministic and probabilistic sensitivity analyses were conducted.
Results: Romiplostim treatment resulted in an average of 20.2 fewer administrations of rescue medication (IVIg or intravenous steroids) over a patient lifetime than eltrombopag, and 29.3 fewer rescue medication administrations than SoC. Romiplostim was dominant, with cost savings of <euro>13,258 and <euro>22,673 and gains of 0.76 and 1.17 quality-adjusted life-years (QALYs), compared with eltrombopag and SoC, respectively. Romiplostim remained cost effective throughout a variety of potential scenarios, including short-term TPOra treatment duration (1 year). One-way sensitivity analysis showed that the model was most sensitive to variation in the cost of IVIg and use of romiplostim and IVIg. Probabilistic sensitivity analysis showed that romiplostim was likely to be cost effective in over 90 % of cases compared with eltrombopag, and 96 % compared with SoC at a willingness-to-pay threshold of <euro>30,000 per QALY.
Conclusions: Use of romiplostim in the ITP treatment pathway, compared with eltrombopag or SoC, is likely to be cost effective in Ireland. Romiplostim improves clinical outcomes by increasing platelet counts, reducing bleeding events and the use of IVIg and steroids, resulting in both cost savings and additional QALYs when compared with current treatment practices.
Figures



Similar articles
-
The Cost-effectiveness of Eltrombopag for the Treatment of Immune Thrombocytopenia in the United States.Clin Ther. 2020 May;42(5):860-872.e8. doi: 10.1016/j.clinthera.2020.02.020. Epub 2020 Mar 18. Clin Ther. 2020. PMID: 32199608
-
Cost-Effectiveness of Eltrombopag versus Romiplostim for the Treatment of Chronic Immune Thrombocytopenia in England and Wales.Value Health. 2016 Jul-Aug;19(5):614-22. doi: 10.1016/j.jval.2016.03.1856. Epub 2016 May 11. Value Health. 2016. PMID: 27565278
-
Cost per response analysis of strategies for chronic immune thrombocytopenia.Am J Manag Care. 2018 Jul;24(8 Spec No.):SP294-SP302. Am J Manag Care. 2018. PMID: 30020741
-
Eltrombopag for the treatment of chronic idiopathic (immune) thrombocytopenic purpura (ITP).Health Technol Assess. 2011 May;15 Suppl 1:23-32. doi: 10.3310/hta15suppl1/03. Health Technol Assess. 2011. PMID: 21609650 Review.
-
[Cost per responder associated with romiplostim and rituximab treatment for adult primary immune thrombocytopenia in France].Transfus Clin Biol. 2014 May;21(2):85-93. doi: 10.1016/j.tracli.2014.03.007. Epub 2014 May 3. Transfus Clin Biol. 2014. PMID: 24797790 French.
Cited by
-
Cost-effectiveness of eltrombopag vs intravenous immunoglobulin for the perioperative management of immune thrombocytopenia.Blood Adv. 2022 Feb 8;6(3):785-792. doi: 10.1182/bloodadvances.2021005627. Blood Adv. 2022. PMID: 34781363 Free PMC article.
-
Cost-effectiveness of adding rituximab to splenectomy and romiplostim for treating steroid-resistant idiopathic thrombocytopenic purpura in adults.BMC Health Serv Res. 2015 Jan 22;15:2. doi: 10.1186/s12913-015-0681-y. BMC Health Serv Res. 2015. PMID: 25609557 Free PMC article.
-
Cost-consequence model comparing eltrombopag and romiplostim in pediatric patients with chronic immune thrombocytopenia.Clinicoecon Outcomes Res. 2018 Nov 5;10:715-721. doi: 10.2147/CEOR.S177338. eCollection 2018. Clinicoecon Outcomes Res. 2018. PMID: 30464564 Free PMC article.
-
Cost-Effectiveness of Thrombopoietin Mimetics in Patients with Thrombocytopenia: A Systematic Review.Pharmacoeconomics. 2023 Aug;41(8):869-911. doi: 10.1007/s40273-023-01271-w. Epub 2023 May 5. Pharmacoeconomics. 2023. PMID: 37145291
-
Is dosing of therapeutic immunoglobulins optimal? A review of a three-decade long debate in europe.Front Immunol. 2014 Dec 12;5:629. doi: 10.3389/fimmu.2014.00629. eCollection 2014. Front Immunol. 2014. PMID: 25566244 Free PMC article. Review.
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura (ITP) of adults and children: report from an International Working Group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. - DOI - PubMed
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

