FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species
- PMID: 23857584
- PMCID: PMC3757174
- DOI: 10.1074/jbc.M113.474106
FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species
Abstract
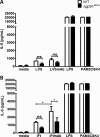
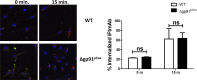
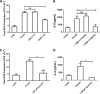
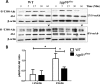
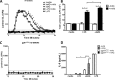
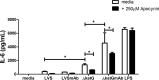
Activation of the FcγR via antigen containing immune complexes can lead to the generation of reactive oxygen species, which are potent signal transducing molecules. However, whether ROS contribute to FcγR signaling has not been studied extensively. We set out to elucidate the role of NADPH oxidase-generated ROS in macrophage activation following FcγR engagement using antigen-containing immune complexes. We hypothesized that NOX2 generated ROS is necessary for propagation of downstream FcγR signaling and initiation of the innate immune response. Following exposure of murine bone marrow-derived macrophages (BMDMs) to inactivated Francisella tularensis (iFt)-containing immune complexes, we observed a significant increase in the innate inflammatory cytokine IL-6 at 24 h compared with macrophages treated with Ft LVS-containing immune complexes. Ligation of the FcγR by opsonized Ft also results in significant ROS production. Macrophages lacking the gp91(phox) subunit of NOX2 fail to produce ROS upon FcγR ligation, resulting in decreased Akt phosphorylation and a reduction in the levels of IL-6 compared with wild type macrophages. Similar results were seen following infection of BMDMs with catalase deficient Ft that fail to scavenge hydrogen peroxide. In conclusion, our findings demonstrate that ROS participate in elicitation of an effective innate immune in response to antigen-containing immune complexes through FcγR.
Keywords: Akt; FC Receptors; Francisella tularensis; Macrophages; NADPH Oxidase; Reactive Oxygen Species (ROS).
Figures


References
-
- Dröge W. (2002) Free Radicals in the Physiological Control of Cell Function. Physiological Rev. 82, 47–95 - PubMed
-
- DeLeo F. R., Allen L.-A. H., Apicella M., Nauseef W. M. (1999) NADPH Oxidase Activation and Assembly During Phagocytosis. J. Immunol. 163, 6732–6740 - PubMed
-
- Fridovich I. (1978) The biology of oxygen radicals. Science 201, 875–880 - PubMed
-
- Sarfstein R., Gorzalczany Y., Mizrahi A., Berdichevsky Y., Molshanski-Mor S., Weinbaum C., Hirshberg M., Dagher M.-C., Pick E. (2004) Dual Role of Rac in the Assembly of NADPH Oxidase, Tethering to the Membrane and Activation of p67phox: A STUDY BASED ON MUTAGENESIS OF p67phox-Rac1 CHIMERAS. J. Biol. Chem. 279, 16007–16016 - PubMed
-
- Bokoch G. M., Diebold B. A. (2002) Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100, 2692–2696 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

