Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers
- PMID: 23857728
- PMCID: PMC5808880
- DOI: 10.1002/prca.201300024
Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers
Abstract
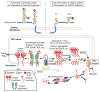
In many different human disorders, the cellular glycome is altered. An interesting but poorly understood alteration occurs in the mucin-type O-glycome, in which there is aberrant expression of the truncated O-glycans Tn (GalNAcα1-Ser/Thr) and its sialylated version sialyl-Tn (STn) (Neu5Acα2,6GalNAcα1-Ser/Thr). Both Tn and STn are tumor-associated carbohydrate antigens and tumor biomarkers, since they are not expressed normally and appear early in tumorigenesis. Moreover, their expression is strongly associated with poor prognosis and tumor metastasis. The Tn and STn antigens are also expressed in other human diseases and disorders, such as Tn syndrome and IgA nephropathy. The major pathological mechanism for expression of the Tn and STn antigens is compromised T-synthase activity, resulting from alteration of the X-linked gene that encodes for Cosmc, a molecular chaperone specifically required for the correct folding of T-synthase to form active enzyme. This review will summarize our current understanding of the Tn and STn antigens in terms of their biochemistry and role in pathology.
Keywords: Cancer; Disease; Glycosylation; IgA nephropathy; Tn antigen.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures




References
-
- Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KT, et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered simple cell lines. Nat Methods. 2011;8:977–982. - PubMed
-
- Ellies LG, Tsuboi S, Petryniak B, Lowe JB, et al. Core 2 oligosaccharide biosynthesis distinguishes between se-lectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. - PubMed
-
- Homeister JW, Thall AD, Petryniak B, Maly P, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. - PubMed
-
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

