Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy
- PMID: 23857747
- PMCID: PMC3893315
- DOI: 10.1002/jbmr.2038
Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy
Abstract
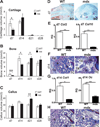
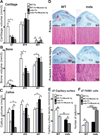
Duchenne muscular dystrophy (DMD) patients exhibit skeletal muscle weakness with continuous cycles of muscle fiber degeneration/regeneration, chronic inflammation, low bone mineral density, and increased risks of fracture. Fragility fractures and associated complications are considered as a consequence of the osteoporotic condition in these patients. Here, we aimed to establish the relationship between muscular dystrophy and fracture healing by assessing bone regeneration in mdx mice, a model of DMD with absence of osteoporosis. Our results illustrate that muscle defects in mdx mice impact the process of bone regeneration at various levels. In mdx fracture calluses, both cartilage and bone deposition were delayed followed by a delay in cartilage and bone remodeling. Vascularization of mdx fracture calluses was also decreased during the early stages of repair. Dystrophic muscles are known to contain elevated numbers of macrophages contributing to muscle degeneration. Accordingly, we observed increased macrophage recruitment in the mdx fracture calluses and abnormal macrophage accumulation throughout the process of bone regeneration. These changes in the inflammatory environment subsequently had an impact on the recruitment of osteoclasts and the remodeling phase of repair. Further damage to the mdx muscles, using a novel model of muscle trauma, amplified both the chronic inflammatory response and the delay in bone regeneration. In addition, PLX3397 treatment of mdx mice, a cFMS (colony stimulating factor receptor 1) inhibitor in monocytes, partially rescued the bone repair defect through increasing cartilage deposition and decreasing the number of macrophages. In conclusion, chronic inflammation in mdx mice contributes to the fracture healing delay and is associated with a decrease in angiogenesis and a transient delay in osteoclast recruitment. By revealing the role of dystrophic muscle in regulating the inflammatory response during bone repair, our results emphasize the implication of muscle in the normal bone repair process and may lead to improved treatment of fragility fractures in DMD patients.
Keywords: ANGIOGENESIS; BONE; CARTILAGE; CHRONIC INFLAMMATION; MACROPHAGE; MUSCLE REGENERATION; MUSCULAR DYSTROPHY; OSTEOCLASTOGENESIS; REGENERATION.
© 2014 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures




References
-
- Sudkamp NP, Haas NP, Sinnig M, Sottmann G, Tscherne H. [Incidence of pseudarthroses in open fractures: analysis of 948 open fractures] Aktuelle Traumatol. 1993;23(2):59–67. PubMed PMID: 8098572. - PubMed
-
- Yazar S, Lin CH, Lin YT, Ulusal AE, Wei FC. Outcome comparison between free muscle and free fasciocutaneous flaps for reconstruction of distal third and ankle traumatic open tibial fractures. Plastic and reconstructive surgery. 2006;117(7):2468–2475. discussion 76-7.PubMed PMID: 16772958. - PubMed
-
- Einhorn TA. The cell and molecular biology of fracture healing. Clinical Orthopaedics and Related Research. 1998;46(355 Suppl):S7–S21. - PubMed

