Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial
- PMID: 23857856
- PMCID: PMC3761040
- DOI: 10.1503/cmaj.121986
Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial
Abstract
Background: Hemorrhage coupled with coagulopathy remains the leading cause of preventable in-hospital deaths among trauma patients. Use of a transfusion protocol with a predefined ratio of 1:1:1 (1 each of red blood cells [RBC], frozen plasma [FP] and platelets) has been associated with improved survival in retrospective studies in military and civilian settings, but such a protocol has its challenges and may increase the risk of respiratory complications. We conducted a randomized controlled trial to assess the feasibility of a 1:1:1 transfusion protocol and its effect on mortality and complications among patients with severe trauma.
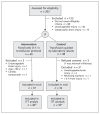
Methods: We included 78 patients seen in a tertiary trauma centre between July 2009 and October 2011 who had hypotension and bleeding and were expected to need massive transfusion (≥ 10 RBC units in 24 h). We randomly assigned them to either the fixed-ratio (1:1:1) transfusion protocol (n = 40) or to a laboratory-results-guided transfusion protocol (control; n = 38). The primary outcome, feasibility, was assessed in terms of blood product ratios and plasma wastage. Safety was measured based on 28-day mortality and survival free of acute respiratory distress syndrome.
Results: Overall, a transfusion ratio of 1:1:1 was achieved in 57% (21/37) of patients in the fixed-ratio group, as compared with 6% (2/32) in the control group. A ratio of 1:1 (RBC:FP) was achieved in 73% (27/37) in the fixed-ratio group and 22% (7/32) in the control group. Plasma wastage was higher with the intervention protocol (22% [86/390] of FP units v. 10% [30/289] in the control group). The 28-day mortality and number of days free of acute respiratory distress syndrome were statistically similar between the groups.
Interpretation: The fixed-ratio transfusion protocol was feasible in our study, but it was associated with increased plasma wastage. Larger randomized trials are needed to evaluate the efficacy of such a protocol in trauma care.
Trial registration: ClinicalTrials.gov, no. NCT00945542.
Figures

Comment in
-
ABO mismatched platelet transfusions in trauma patients.CMAJ. 2014 Jan 7;186(1):63. doi: 10.1503/cmaj.114-0001. CMAJ. 2014. PMID: 24396123 Free PMC article. No abstract available.
References
-
- Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion 2006;46:685–6 - PubMed
-
- Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 2007;62:307–10 - PubMed
-
- Zink KA, Sambasivan CN, Holcomb JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 2009;197:565–70 - PubMed
-
- Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007; 63:805–13 - PubMed
-
- Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma 2008;64:1177–82 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
