Ku70 and non-homologous end joining protect testicular cells from DNA damage
- PMID: 23857907
- PMCID: PMC3711201
- DOI: 10.1242/jcs.122788
Ku70 and non-homologous end joining protect testicular cells from DNA damage
Abstract
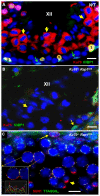
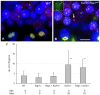
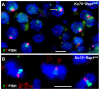
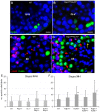
Spermatogenesis is a complex process that generates haploid germ cells or spores and implements meiosis, a succession of two special cell divisions that are required for homologous chromosome segregation. During prophase to the first meiotic division, homologous recombination (HR) repairs Spo11-dependent DNA double-strand breaks (DSBs) in the presence of telomere movements to allow for chromosome pairing and segregation at the meiosis I division. In contrast to HR, non-homologous end joining (NHEJ), the major DSB repair mechanism during the G1 cell cycle phase, is downregulated during early meiotic prophase. At somatic mammalian telomeres, the NHEJ factor Ku70/80 inhibits HR, as does the Rap1 component of the shelterin complex. Here, we investigated the role of Ku70 and Rap1 in meiotic telomere redistribution and genome protection in spermatogenesis by studying single and double knockout mice. Ku70(-/-) mice display reduced testis size and compromised spermatogenesis, whereas meiotic telomere dynamics and chromosomal bouquet formation occurred normally in Ku70(-/-) and Ku70(-/-)Rap1(Δ/Δ) knockout spermatocytes. Elevated mid-preleptotene frequencies were associated with significantly increased DNA damage in Ku-deficient B spermatogonia, and in differentiated Sertoli cells. Significantly elevated levels of γH2AX foci in Ku70(-/-) diplotene spermatocytes suggest compromised progression of DNA repair at a subset of DSBs. This might explain the elevated meiotic metaphase apoptosis that is present in Ku70-deficient stage XII testis tubules, indicating spindle assembly checkpoint activation. In summary, our data indicate that Ku70 is important for repairing DSBs in somatic cells and in late spermatocytes of the testis, thereby assuring the fidelity of spermatogenesis.
Keywords: Bouquet formation; DNA damage; Ku70; Meiosis; Mid-preleptotene; NHEJ; Rap1; Recombination; Sertoli cell; Shelterin; Spermatogenesis; Telomere attachment.
Figures



References
-
- Ahmed E. A., van der Vaart A., Barten A., Kal H. B., Chen J., Lou Z., Minter-Dykhouse K., Bartkova J., Bartek J., de Boer P. et al.(2007). Differences in DNA double strand breaks repair in male germ cell types: lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair (Amst.) 6, 1243–1254 10.1016/j.dnarep.2007.02.011 - DOI - PubMed
-
- Ahmed E. A., Barten-van Rijbroek A. D., Kal H. B., Sadri-Ardekani H., Mizrak S. C., van Pelt A. M., de Rooij D. G. (2009). Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol. Reprod. 80, 1084–1091 10.1095/biolreprod.108.071662 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

